Comparison of Contrast Enhanced MDCT and Integrated FDG PET/CT Staging of Colorectal Cancer
Manavjit S Sandhu, Divya Singh, Naveen Kalra, Anmol Bhatia, Anish Bhattacharya, Rajesh Gupta and Niranjan Khandelwal
Manavjit S Sandhu1, Divya Singh1 Naveen Kalra1*, Anmol Bhatia1, Anish Bhattacharya1, Rajesh Gupta2 and Niranjan Khandelwal1
1Departments of Radiodiagnosis and Imaging, Nuclear Medicine, Post Graduate Institute of Medical Education and Research (PGIMER), Chandigarh, India
2Departments of Radiodiagnosis and Imaging, General Surgery, Post Graduate Institute of Medical Education and Research (PGIMER), Chandigarh, India
- *Corresponding Author:
- Dr. Naveen Kalra, Professor, Department of Radiodiagnosis and Imaging, Post Graduate Institute of Medical Education and Research PGIMER), Chandigarh, India.
Tel: (954)-214-3743
E-mail: navkal2004@yahoo.com
Received date: February 06, 2016; Accepted date: February 17, 2016; Published date: February 23, 2016
Citation: Sandhu MS, Singh D, Kalra N, et al. Comparison of Contrast Enhanced MDCT And Integrated FDG PET/CT Staging of Colorectal Cancer. Colorec Cancer 2016, 2:2. doi: 10.21767/2471-9943.100017
Abstract
Introduction: To compare contrast enhanced multidetector computed tomography (CEMDCT) with positron emission tomography/computed tomography (PET/CT) in staging of primary colorectal carcinoma. Methods: This was an institute review board approved prospective study done over a period of 18 months. 15 patients of histologically proven colorectal carcinoma on endoscopy were included. Scanning was performed in 3D mode on an integrated PET/ CT system. Initially, CEMDCT was performed from head to mid thigh. Following this, PET scan was acquired. Pre-operative TNM staging was done by both PET/CT and CEMDCT scans. The findings were correlated with the operative and histopathological findings which were considered standards of reference, and used for calculating the accuracy. All patients underwent surgery and histopathological staging was obtained. One patient had 2 synchronous malignant lesions. Hence, a total of 16 colorectal cancers were evaluated.
Results: The overall diagnostic accuracy for tumor, node and metastasis (TNM) staging on CEMDCT was 68.75%, 50% and 87.5% for primary tumor, regional lymph node, and distant metastasis, respectively. The overall diagnostic accuracy for TNM staging on PET/CT was 87.5%, 31.25% and 87.5% for primary tumor, regional lymph node, and distant metastasis, respectively. A higher accuracy of primary tumor staging was seen with PET/CT while accuracy for detection of distant metastasis was same with both modalities. On CEMDCT M0 stage had one false negative while no false negative seen on PET/CT. Accuracy for regional lymph node staging was poor with both modalities, although it was better with CEMDCT. There was slight agreement between the CEMDCT and PET/CT with Kendal l’s tau-b test showing value of 0.403. ‘p’ value was 0.052, suggesting not a significant relationship level. Kappa value was 0.162 which shows that there was a poor strength of agreement.
Conclusion: In view of higher accuracy of primary tumor staging, PET/CT can be considered as a first line investigation for preoperative evaluation of patients with colorectal cancer.
Keywords
Colorectal cancer; PET; CT; CEMDCT; Staging
Introduction
Colorectal cancer is amongst the leading cancers worldwide. Globally, the age standardized rate (per 100,000) of colorectal cancer in males is 19.1 and in females is 14.4 [1]. In the Indian population, the incidence rates of colon cancer varies from 0.7 to 3.7/100,000 among males and 0.4 to 3/100,000 among females as reported in eight population registries. The incidence rates (per 100,000) in rectal cancer ranges from 1.6 to 5.5 in males and 0 to 2.8/100,000 in females [2].
The preoperative imaging aims at determining the extent of the primary tumor, contiguous organ involvement, distant metastases or synchronous malignancy. This enables accurate staging which is of utmost importance in planning treatment, determining prognosis, and subsequent follow up of the patient.
Computed tomography (CT) is an established method in staging the involvement of the liver and periluminal tumor extension. CT has been shown to have an accuracy of 74% in assessment of wall invasion and a sensitivity of 48% for lymph node metastases. For detection of liver metastases, CT has been shown to have an accuracy of 85% and a specificity of 97%. The major limitation of CT was related to its size criteria for differentiating benign from malignant lymph nodes [3]. Three dimensional spiral CT combined with virtual endoscopy is a potential alternative screening technique to colonoscopy for detection of polypoidal colonic lesions.
Positron emission tomography (PET) has an increased sensitivity for the detection of abdominal lymph nodes. 18F-FDG-PET is also accurate for evaluation of liver metastases [4]. Lack of detection of some small metastases and possible false-positive findings due to normal gastrointestinal tract uptake and in lesions containing activated macrophages account for the major limitations of PET. However, 18-FDG-PET is an invaluable imaging modality for detecting recurrence at the postsurgical site. The advantage of PET/CT is that it combines the functional and anatomical data of the two modalities. Clinically, the important additional information provided by PET/CT is accuracy in the detection of distant metastases [4].
There are very few studies in literature comparing CT with PET/ CT in staging of primary colorectal carcinoma, none of them from India. It was in this context that the present study was proposed.
Materials and Methods
This was an institute review board approved prospective study conducted in a tertiary care centre of northern India over a period of 18 months. 15 patients of histologically proven colorectal carcinoma on sigmoidoscopy/ colonoscopy were included in our study. Informed consent was obtained in all cases.
Patients with contraindication for radiographic contrast like acute/chronic renal insufficiency and documented history of allergy to iodinated contrast media, who had undergone surgery for colorectal cancer and patients who didn’t give consent for being included in the study were excluded.
MDCT protocol
Multi detector computed tomography (MDCT) scanning was done as a part of the PET/CT protocol. Initially, MDCT was performed on Discovery STE16 (GE Medical Systems, Milwaukee) from the head to the mid thigh with parameters of 120 kV, 200-250 mA, tube rotation time of 0.5 s per rotation (pitch 6); 16×0.75mm collimation, table feed of 22.5mm per rotation and section thickness of 10 mm. Prior to scanning, 40 ml of ionic contrast (Diatrizoate Sodium) diluted in 2 litres of water was given to the patient to drink over a period of 2.5 hours. A power injector (Mallinckrodt; Optivantage DH) was used to inject 100ml of contrast medium (Iohexol 300 mg/ml; Omnipaque®, GE Healthcare) intravenously at a rate of 3 ml/s. CT was performed in the portal-venous phase with a 70 second delay between the start of contrast material administration and the start of helical scanning. The 10 mm-thick transverse CT images were reconstructed at 2.5 mm intervals for interpretation of MDCT data.
PET protocol
All the patients were fasting for at least 12 hours prior to the examination. The blood sugar levels of the patients were determined before the examination. Patients with blood sugar levels more than 150 mg/dl were excluded as glucose competes with 18F-FDG for cellular uptake. Fifty minutes after the intravenous injection of 370 MBq (10 mCi) FDG, the scan was started in 3D mode (2 minutes per bed position). Scanning was performed in a 3D mode on an integrated PET/CT system (Discovery STE16, GE Medical Systems, Milwaukee). Emission data was acquired for 5–7 bed positions. The time at each bed position was kept at 3 minutes. The 18F-FDG-PET images were reconstructed using an iterative reconstruction algorithm (ordered subsets expectation maximization).
PET/CT
The MDCT data was reconstructed to a slice thickness of 3.8mm so that it could be used for attenuation correction and was fused with the FDG-PET images to obtain the PET/CT images.
Image interpretation
Patients’ image data sets were separated into MDCT and PET/ CT data sets. The MDCT data was interpreted separately by a radiologist (more than 10 years of experience in abdominal imaging) who was blinded to the PET and PET/CT findings. The PET/CT images were interpreted by a nuclear medicine physician (more than seven years of experience) using an Advantage Windows® (GE Medical Systems, Milwaukee) workstation. A lesion-by-lesion analysis was performed. Pre-operative TNM staging was done by both PET/CT and MDCT scans. The findings of these investigations were correlated with the operative and histopathological findings. In patients with rectal carcinoma, who were given neoadjuvant chemo-radiation, the imaging was done 6 weeks after completion of chemo-radiation therapy.
Standard of reference
The standards of reference for staging were surgery and histopathological findings of surgical specimen. Surgery was done within 2 weeks after MDCT and PET/CT. For the depth of tumor invasion (T) and nodal involvement (N), pathological findings were the reference standard. The reference standard for liver, peritoneum and retroperitoneal metastases were the intraoperative findings, surface exploration and histological/ cytological examination, which were used for calculating the accuracy. All the 15 patients underwent surgery and histopathological staging was obtained which was correlated with contrast enhanced MDCT and PET/CT staging. In our study, one patient had 2 synchronous malignant lesions. Hence, a total of 16 colorectal cancers were evaluated.
Statistical analysis
Statistical analysis was done using SPSS version 17software. Continuous variables were expressed as mean ± standard deviation and categorical values as frequency. Statistical analysis was done independently between CEMDCT and PET/CT with surgical and histopathological TNM staging by using Kendall’s tau-b test. Kappa value (measure of agreement) was also calculated. Level of significance was obtained and p value <0.05 was considered to be significant. Diagnostic accuracy of TNM staging was also calculated.
Results
This was a prospective study done over a period of 18 months. Fifteen patients (10 males and 5 females) were enrolled in the study. The mean age group was 51.47±13.53 years and median was 55 years.
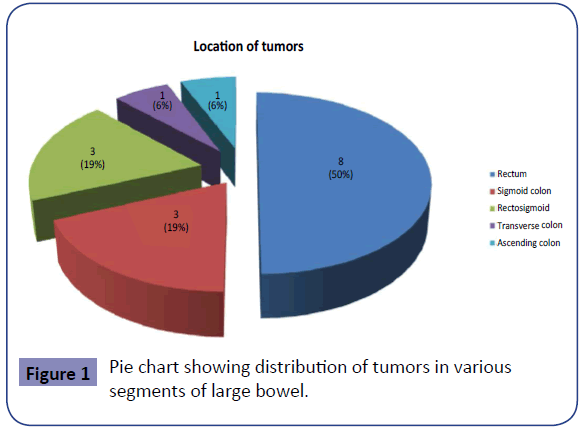
Most of the patients presented with per rectal bleeding (80%), altered bowel habits (73.3%), loss of weight (73.3%), loss of appetite and generalized weakness (53.3%). Only four patients (26.7%) presented with pain abdomen. One patient (6.7%) each had lump abdomen and fever. CEA levels were obtained in all 15 patients. 4.7ng/ml was taken as the cut-off for normal CEA level. It was seen that 46.7% of the patients had CEA levels < 4.7 ng/ ml (range: 2.48 – 4.5 ng/ml) and in 53.3%, the CEA levels were elevated (range: 5.4 – 17.4 ng/ml). Figure 1 shows the distribution of 16 tumors in the various segments of the bowel.
Majority of the tumors showed asymmetric mural thickening (81.25%). Polypoidal morphology was seen in 31.25% of the patients. Only asymmetric mural thickening was seen in 11 (68.75%) tumors, while only polypoidal morphology in 3 (18.75%) tumors. Two (12.5%) tumors had both asymmetric mural thickening and polypoidal morphology.
TNM staging on CEMDCT
T1 and T2 stages cannot be reliably differentiated on CEMDCT and were considered as one category, i.e., ≤T2 when bowel wall thickness exceeded 5mm. 6.25% of the tumors were placed in this category. Majority of the tumors were classified as T3 stage (68.75%) because of presence of pericolonic stranding and/or advancing nodular margin. 25% of the tumors were classified as T4 stage due to loss of fat planes with adjacent organs or structures.
43.75% of the tumors were not associated with any pericolonic/ perirectal lymphadenopathy and were labeled as N0.1-3 pericolic/perirectal nodes were seen in 50% of the tumors and were labelled as N1. 4 or more pericolic/ perirectal nodes were seen in 6.25% of the tumors and were labelled as N2.
Out of 16 tumors, distant haematogenous metastases (M1) were seen in 3 (18.75%). Two of these were in the liver. There was no distant metastasis (M0) in 13 (81.25%) tumors. TNM staging of the tumors on CEMDCT is summarized in Table 1.
| TNM Staging | No.of tumors [n=16] | Percentage |
|---|---|---|
| Stage 0 (Tis N0M0) | 0 | 0 |
| Stage I (T1, 2 N0 M0) | 1 | 6.25% |
| Stage IIA (T3 N0 M0) | 3 | 18.75% |
| Stage IIB (T4 N0 M0) | 2 | 12.5% |
| Stage IIIA (T1, T2 N1 M0) | 0 | 0 |
| Stage IIIB (T3, T4 N1 M0) | 6 | 37.5% |
| Stage IIIC (Any T N2 M0) | 1 | 6.25% |
| Stage IV (Any T Any N M1) | 3 | 18.75% |
| Total | 16 | 100% |
*T: Tumor
N: Node
M: Metastasis
** CEMDCT: Contrast enhanced multi detector computed tomography
Table 1: TNM* staging on CEMDCT**
TNM staging on PET/CT
One (6.25%) tumor was placed in T0 category in view of nonidentification of primary tumor. Majority of the tumors were classified as T3 81.25% because of presence of pericolonic stranding and/or advancing nodular margin. Two (12.5%) of the tumors were classified as T4 stage due to loss of fat planes with adjacent organs or structures.
Five (31.25%) of the tumors were not associated with any pericolonic/ perirectal lymphadenopathy and were labeled as N0. 1-3 pericolic / perirectal FDG avid nodes were seen in 10 (62.5%) of the tumors and were labelled as N1. Four or more pericolic/ perirectal FDG avid nodes were seen in (6.25%) of the tumors and were labelled as N2.
On PET/CT, out of 16 tumors, distant metastases M1) were seen in 3 (18.75%). There was no distant metastasis M0) in (13 81.25%) of the tumors. Table 2 summarizes TNM staging on PET/CT.
| TNM Staging | No .of tumors[n=16] | Percentage |
|---|---|---|
| Stage 0 (Tis N0M0) | 1 | 6.25% |
| Stage I (T1, 2 N0 M0) | 0 | 0 |
| Stage IIA (T3 N0 M0) | 3 | 18.75% |
| Stage IIB (T4 N0 M0) | 0 | 0 |
| Stage IIIA (T1, T2 N1 M0) | 0 | 0 |
| Stage IIIB (T3, T4 N1 M0) | 8 | 50% |
| Stage IIIC (Any T N2 M0) | 1 | 6.25% |
| Stage IV (Any T Any N M1) | 3 | 18.75% |
| Total | 16 | 100% |
*PET/CT: Positron emission tomography/computed tomography
Table 2: TNM Staging on PET/CT*
TNM staging on surgery
One (6.25%) tumor was placed in T0 category in view of nonidentification of primary tumor. Majority of the tumors were classified as T3 (68.75%) because of invasion through muscularis propria into subserosa or into non peritonealized pericolic or perirectal tissues. Two (12.5%) tumors were classified as T4 stage due to direct invasion of other organs.
14 (87.5%) of the tumors were not associated with any pericolonic/ perirectal lymphadenopathy and were labeled as N0. 1-3 pericolic / perirectal lymph nodes were seen in 2 (12.5%) of the tumors and were labelled as N1. No tumor was labeled as stage N2.
On surgery, out of 16 tumors, distant hematogenous metastases (M1) were seen in 3 (18.75). There was no distant metastasis (M0) in 13 (81.25%) of the tumors. Table 3 summarizes the TNM staging of the tumors on surgery.
| TNM Staging | No.of tumors[n=16] | Percentage |
|---|---|---|
| Stage 0 (Tis N0M0) | 1 | 6.25% |
| Stage I (T1, 2 N0 M0) | 2 | 12.5% |
| Stage IIA ( T3 N0 M0 ) | 8 | 50% |
| Stage IIB (T4 N0 M0) | 2 | 12.5% |
| Stage IIIA (T1, T2 N1 M0) | 0 | 0 |
| Stage IIIB ( T3, T4 N1 M0 ) | 0 | 0 |
| Stage IIIC (Any T N2 M0) | 0 | 0 |
| Stage IV (Any T Any N M1) | 3 | 18.75% |
| Total | 16 | 100% |
Table 3: TNM Staging on surgery.
TNM staging on histopathology
One (6.25%) tumor was placed in T0 category in view of nonidentification of primary tumor. Majority of the tumors were classified as T3 (68.75%) because of invasion through muscularis propria into subserosa or into non peritonealized pericolic or perirectal tissues. 2 (12.5%) of the tumors were classified as T4 stage due to direct invasion of other organs.
15 (93.75%) of the tumors were not associated with any pericolonic/ perirectal lymphadenopathy and were labeled as N0. 1-3 pericolic / perirectal lymph nodes were seen in 1 (6.25%) of the tumors and were labelled as N1. No tumor was labeled as stage N2.
On histopathology, out of 16 tumors, distant haematogenous metastases (M1) were seen in 2 (12.5%). There was no distant metastasis M0) in 14 (87.5%) of the tumors. Table 4 summarizes the TNM staging of tumors on histopathology.
| TNM Staging | No. of tumors[n=16] | Percentage |
|---|---|---|
| Stage 0 (Tis N0M0) | 1 | 6.25% |
| Stage I (T1, 2 N0 M0) | 2 | 12.5% |
| Stage IIA (T3 N0 M0) | 8 | 50% |
| Stage IIB (T4 N0 M0) | 2 | 12.5% |
| Stage IIIA (T1, T2 N1 M0) | 0 | 0 |
| Stage IIIB (T3, T4 N1 M0) | 1 | 6.25% |
| Stage IIIC (Any T N2 M0) | 0 | 0 |
| Stage IV (Any T Any N M1) | 2 | 12.5% |
| Total | 16 | 100% |
Table 4: TNM staging on histopathology.
Correlation between CEMDCT and surgical staging
There was slight agreement between the two modalities with Kendall’s tau-b test showing value of 0.379. ‘p’ value was 0.05, suggesting not significant relationship level. Kappa value was 0.170 which shows that there is poor strength of agreement.
Correlation between CEMDCT and histopathological staging
On comparison of the two modalities, Kendall’s tau-b test showed a value of 0.396. ‘p’ value was 0.049, which was significant. Kappa was also calculated which was 0.178, the strength of agreement being poor.
Correlation between PET/CT and surgical staging [n=16]
There was slight agreement between the two modalities with Kendall’s tau-b test showing value of 0.092. ‘p’ value was 0.76 which was not significant. The value of kappa was 0.356, suggesting the strength of agreement to be fair.
Correlation between PET/CT and Histopathological staging
There was little agreement between the two modalities with Kendall’s tau-b test showing a value of 0.457. ‘p’ value was 0.088, suggesting non-significance. There was fair agreement between the two modalities with kappa value of 0.308.
Correlation between CEMDCT and PET/CT staging
There was slight agreement between the two modalities with Kendall’s tau-b test showing value of 0.403. ‘p’ value was 0.052, suggesting not significant relationship level. Kappa value was 0.162 which shows that there is poor strength of agreement.
Diagnostic accuracy
The overall diagnostic accuracy for TNM staging of colorectal cancer in our study on CEMDCT was 68.75%, 50% and 87.5% for primary tumor, regional lymph node, and distant metastasis, respectively. The overall diagnostic accuracy for TNM staging of colorectal cancer in our study on PET/CT was 87.5%, 31.25% and 87.5% for primary tumor, regional lymph node, and distant metastasis, respectively (Figures 2-5).
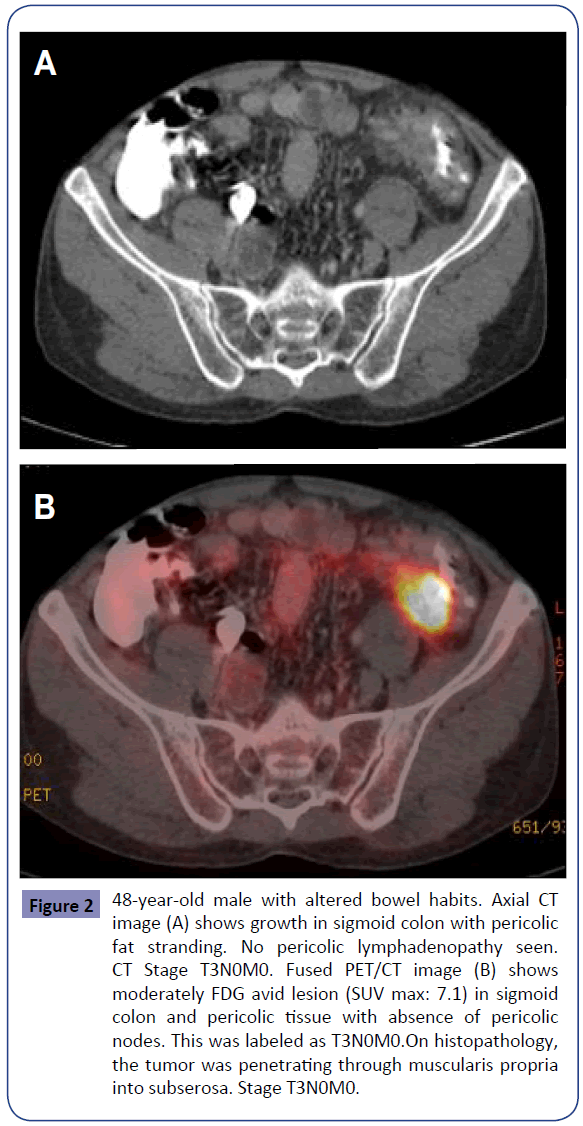
Figure 2: 48-year-old male with altered bowel habits. Axial CT image (A) shows growth in sigmoid colon with pericolic fat stranding. No pericolic lymphadenopathy seen. CT Stage T3N0M0. Fused PET/CT image (B) shows moderately FDG avid lesion (SUV max: 7.1) in sigmoid colon and pericolic tissue with absence of pericolic nodes. This was labeled as T3N0M0.On histopathology, the tumor was penetrating through muscularis propria into subserosa. Stage T3N0M0.
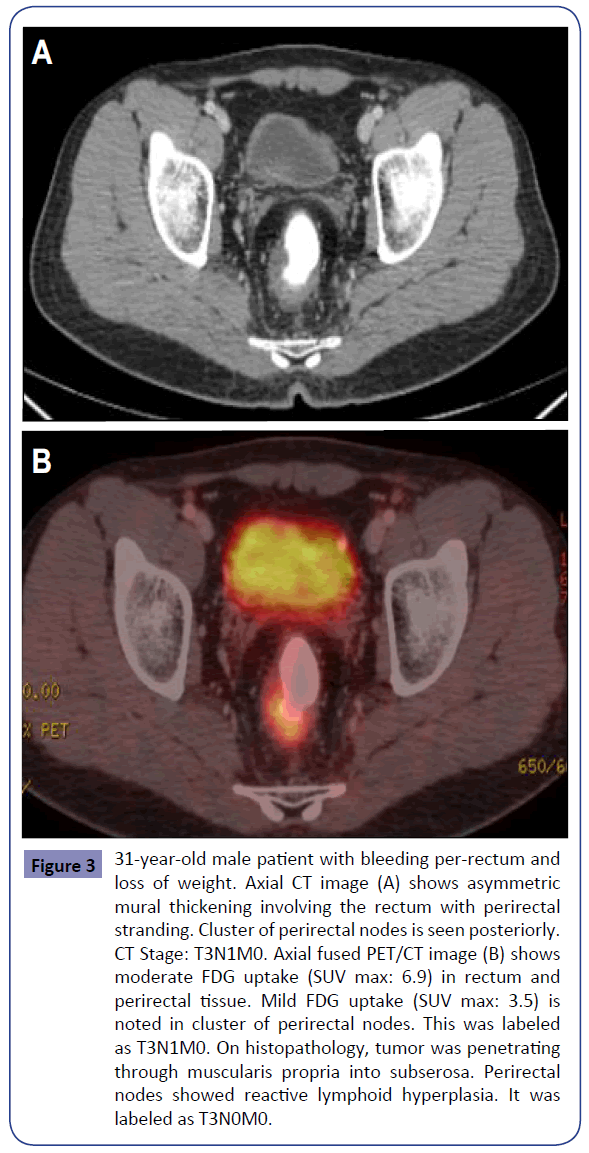
Figure 3: 31-year-old male patient with bleeding per-rectum and loss of weight. Axial CT image (A) shows asymmetric mural thickening involving the rectum with perirectal stranding. Cluster of perirectal nodes is seen posteriorly. CT Stage: T3N1M0. Axial fused PET/CT image (B) shows moderate FDG uptake (SUV max: 6.9) in rectum and perirectal tissue. Mild FDG uptake (SUV max: 3.5) is noted in cluster of perirectal nodes. This was labeled as T3N1M0. On histopathology, tumor was penetrating through muscularis propria into subserosa. Perirectal nodes showed reactive lymphoid hyperplasia. It was labeled as T3N0M0.
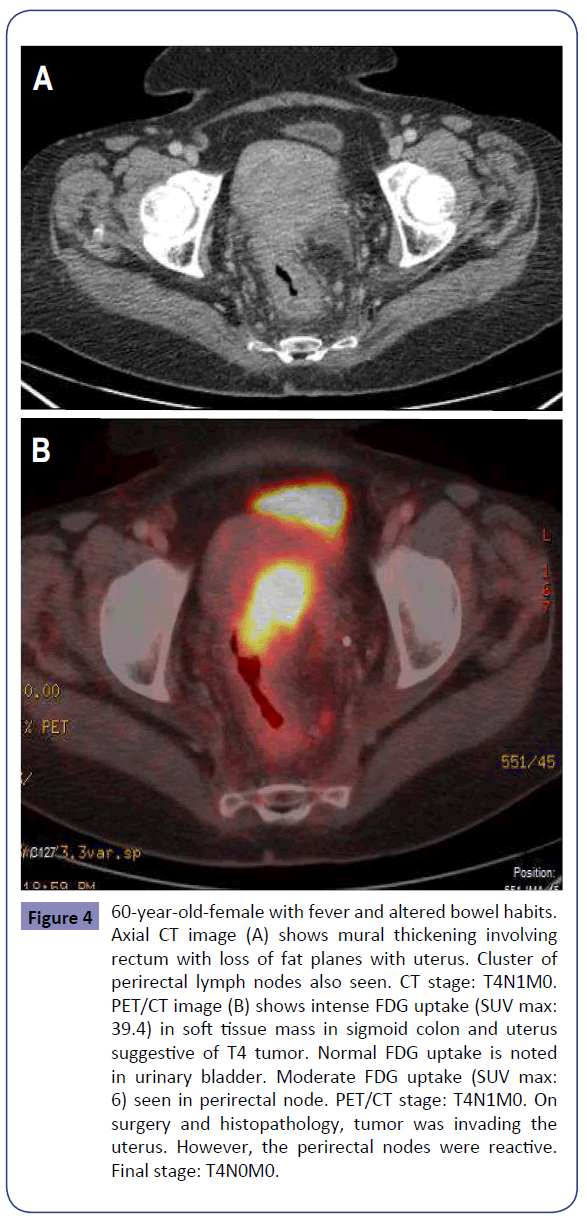
Figure 4: 60-year-old-female with fever and altered bowel habits. Axial CT image (A) shows mural thickening involving rectum with loss of fat planes with uterus. Cluster of perirectal lymph nodes also seen. CT stage: T4N1M0. PET/CT image (B) shows intense FDG uptake (SUV max: 39.4) in soft tissue mass in sigmoid colon and uterus suggestive of T4 tumor. Normal FDG uptake is noted in urinary bladder. Moderate FDG uptake (SUV max: 6) seen in perirectal node. PET/CT stage: T4N1M0. On surgery and histopathology, tumor was invading the uterus. However, the perirectal nodes were reactive. Final stage: T4N0M0.
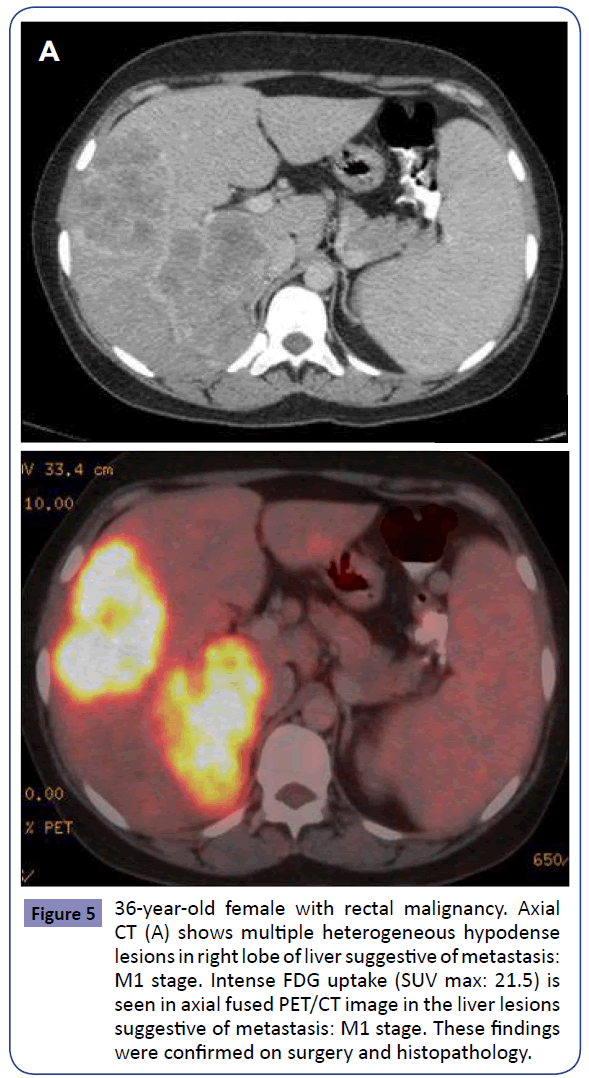
Figure 5: 36-year-old female with rectal malignancy. Axial CT (A) shows multiple heterogeneous hypodense lesions in right lobe of liver suggestive of metastasis: M1 stage. Intense FDG uptake (SUV max: 21.5) is seen in axial fused PET/CT image in the liver lesions suggestive of metastasis: M1 stage. These findings were confirmed on surgery and histopathology.
Discussion
PET/CT has evolved as a novel, promising technique which provides functional information of FDG-PET along with superior spatial and contrast resolution of MDCT. By virtue of providing this dual information it may serve as a one stop investigation in the pre- operative staging of colorectal cancer, thereby eliminating the need of multiple modalities for staging. For patients with colorectal cancer, stage is the strongest predictor of survival [5,6]. Through this study, we have compared the diagnostic accuracy of staging of colorectal cancers using CEMDCT and PET/CT with surgical and histopathological staging.
The overall diagnostic accuracy for TNM staging of colorectal cancer in our study on CEMDCT was 68.75%, 50% and 87.5% for primary tumor, regional lymph node, and distant metastasis, respectively. Theoni et al showed an overall staging accuracy of CT of around 92% [7]. Balthazar et al. showed a sensitivity of 55% for local invasion, 73% for regional nodes, and 79% for liver metastases by CT [8]. Acunas et al. found that for detection of extramural invasion CT had a sensitivity and specificity of 60% and 67% respectively, sensitivity and specificity of 75% for lymph node metastases, and a sensitivity of 87% and specificity of 95% for liver metastases [9]. Angelelli et al. showed that accuracy of CT in the assessment of local invasion was 97.6%, while in the detection of lymph node involvement, it was 78.6% [10]. Chiesura- Corona et al evaluated radiologic T and N staging which was compared with pathologic examination of excised specimens. CT correctly staged 86/105 (82%) lesions. Overestimation occurred in T2 patients (11/61, 18%) and underestimation in T3 patients (7/33, 21%). The criteria suggested for evaluating metastatic perirectal lymph nodes (diameter >5 mm) provided a diagnostic accuracy of 79.2%, sensitivity of 88.5%, and negative predictive value of 86.5% [11].
In our study, a positive correlation was obtained between CEMDCT and surgical staging of ‘T’ staging of 16 tumors (k value: 0.61) and between CEMDCT and histopathological staging of 16 tumors (k value: 0.71) and ‘p’ value was <0.008, suggesting high level of significance in both studies. However, a stronger correlation was seen with histopathology as shown by the kappa values.
One primary tumor in the rectosigmoid region was the lead point of an intussusception which was confirmed on surgery. Eleven T3 tumors were reported on CEMDCT which showed 81.8% correlation with histopathology and surgery as both surgery and histopathology downstaged two tumors to T2 stage. On CEMDCT four tumors were reported as T4. Two of them were confirmed on surgery and histopathology. However, in two cases loss of fat planes with the adjacent organs was secondary to adhesions and adjacent reactive changes and no direct tumor infiltration was demonstrated on both surgery and histopathology. The change in contour and attenuation of the neighboring viscera can be a more reliable sign of tumor infiltration. In one case of T4 tumor, there was loss of fat planes with the ileum. In the second case, there was loss of fat plane with the seminal vesicle. Thus, mere loss of fat plane was not diagnostic of infiltration of an adjacent organ in our study.
In seven cases, no pericolonic lymphadenopathy was reported in CEMDCT which was confirmed on histopathology suggesting a negative predictive value of 100% for lymph node detection. However out of the seven cases reported as N0 stage on CEMDCT, six cases correlated with surgery and one was reported as N1. Out of 8 cases reported as N1 on CEMDCT, both surgery and histopathology confirmed only one case as true N1 stage. The single CEMDCT N2 case was reported as N0 both in surgery and histopathology. The histopathological finding in the tumors suspected to have regional lymph nodal metastasis was reactive lymphoid hyperplasia. This implied that size criteria (nodes>1cm in long-axis), even if clubbed with other factors, such as number and cluster cannot distinguish malignant nodes from enlarged (>1 cm) reactive benign nodes [12].
Three cases were reported on CEMDCT as M1 stage. Two of these were liver metastases, which were confirmed both on surgery and histopathology. Of the 13 cases stated as M0, one false negative was identified on surgery while no distant metastasis was reported on histopathology suggesting a negative predictive value of 100% for distant metastasis detection.
Shin et al (2008) reviewed the role of CT and integrated FDG PET/CT for preoperative staging of colorectal cancer and found a primary detection rate of PET/CT between 95-100%. However, it was difficult to differentiate T2 from T3, and T3 from T4 tumors [13]. Dirisamer et al. evaluated the role of PET/CT for adding information over PET or contrast-enhanced CT alone for staging/ restaging the patients with colorectal cancer. Per- lesion sensitivity with CE PET/CT, CECT and PET were found to be 100%, 91% and 85% respectively. PET/CT identified 2 false positive lesions. PET/ CT also had a per- patient sensitivity of 100%, which was superior to CE CT and PET [14].
Mainenti et al. evaluated the accuracy of PET/CT in assessment of T stage of colorectal cancer. In their study of 34 consecutive patients, colorectal wall invasion was analysed according to a modified T classification that considered three stages (<T2, T3, T4) only. It was found that PET/CT correctly staged the T of 33 out of 35 lesions identified, showing an accuracy of 94.3%. All T1, T3 and T4 lesions were correctly staged, while two T2 neoplasms, located in the sigmoid colon and rectum, respectively, were overstaged as T3 [15]. In our study, a moderately positive correlation was obtained between PET/CT and surgical staging of ‘T’ staging of 16 tumors (k value: 0.551) while good correlation was seen between PET/CT and histopathological staging of 16 tumors (k value: 0.704) and ‘p’ value was 0.021, suggesting a high level of significance in both studies. However, a stronger correlation was seen with histopathology as shown by the kappa values.
Eleven T3 tumors were reported on PET/CT which showed 84.6% correlation with histopathology and surgery as both surgery and histopathology downstaged two tumors to T2 stage. Two tumors were reported as T4 on PET/CT. Both of them were confirmed on surgery and histopathology. By taking advantage of the functional information provided by PET/CT, it correctly identified two T3 tumors which were overstaged as T4 on CEMDCT as the adjacent organs which appeared to be infiltrated on MDCT but did not show any evidence of FDG uptake on PET/CT. This accounted for the higher accuracy of PET/ CT in T staging (87.5%) vis-a-vis that of CEMDCT (68.75%).
Shin et al observed that PET/CT had a low sensitivity (43%) and a high specificity (80%) for regional nodal metastasis suggesting that PET/CT preoperatively had a limited value for detecting metastasis to the regional lymph nodes. Mianenti et al. [13] showed accuracy of 79.4% of PET/CT in N staging [15]. In our study, in four cases no pericolonic lymphadenopathy was reported in PET/CT which was confirmed on both surgery and histopathology suggesting a negative predictive value of 100% for lymph node detection.
However out of eleven cases reported as N1 stage on PET/CT, surgery confirmed only two cases and histopathology confirmed only one case as true N1 stage. The single PET/CT reported N2 case was reported as N0 both with surgery and histopathology. The histopathological finding in the tumors reported as N0 was reactive lymphoid hyperplasia. This suggests that mere FDG avidity of lymph nodes does not qualify them to be infiltrated by tumor cells. Lymph nodes enlarged secondary to inflammation can also show significant FDG uptake thereby mimicking malignant deposits leading to a diagnostic dilemma. It is postulated that the large number of FDG avid nodes which showed reactive lymphoid hyperplasia on histopathology and led to poor diagnostic accuracy of PET/CT (31.25%) could be due to high prevalence of chronic granulomatous infections in the Indian scenario.
One of the lymph nodes with a maximum SUV of 6.1 (Image 10) was found to have granulomatous inflammation. This highlights the fact that the SUV in isolation is not specific for lymph node involvement by tumor cells and it can be raised even in the setting of inflammation. Therefore, SUV cannot help in differentiating benign from malignant lymph nodes. Its primary utility may be in establishing a baseline value prior to initiation of neoadjuvant therapy and later evaluating response to treatment by observing the change in SUV. Also, it can serve as a guiding tool in deciding which lymph node should be sampled for better diagnostic yield in case of multiple lymph nodal enlargements.
In our study, 3 cases were reported on PET/CT as M1 stage. Two of them were confirmed as liver metastasis both on surgery and histopathology. The third tumor in the rectosigmoid region staged as M1 on PET/CT had FDG avid periportal lymph nodes. However, on histopathological examination these turned out to be reactive lymphoid hyperplasia. Thus, PET/CT had 100% positive predictive value in detection of liver metastasis. Histopathology correlated 100% with PET/CT for M0 stage. This showed that PET/CT had 100% negative predictive value for distant metastasis. Shin et al showed that in M staging, CE-PET/CT can be used a onestep diagnostic procedure to detect hepatic metastases. There was marked improvement in the certainty of localization and characterization of FDG-avid lesions, especially in identification of extrahepatic disease (sensitivity of 89% versus 64% of CT) [13].
Eleven T3 tumors were reported on CEMDCT which showed 100% correlation with PET/CT. On CEMDCT four tumors were reported as T4. Two of them were confirmed on PET/CT. However, in two cases loss of fat planes with adjacent organs was secondary to adhesions and adjacent reactive changes and PET/CT downgraded them to T3 which was confirmed on surgery and histopathology. In one case of T4 tumor, there was loss of fat planes with the ileum while in the second case, there was loss of fat plane with the seminal vesicle.
Out of the seven cases in which no pericolonic lymphadenopathy was reported in CEMDCT, six were reported as N1 on PET/CT. Out of eight cases reported as N1 stage on CEMDCT, only four cases correlated with PET/CT and four were reported as N0. The single CEMDCT reported N2 case was also reported as N2 by PET/CT.
Three cases were reported on CEMDCT as M1 stage. Two of them were reported as M1 on PET/CT. These were metastasis in the liver. Of the 13 cases stated as M0 on CEMDCT, 12 were reported as M0 on PET/CT while one was reported as M1. This was a case with subcentimetric periportal nodes which were intensely avid on PET/CT.
We think that the small study group is a major limitation of our study. More studies in future with larger patients could provide more representative results.
Conclusion
The comparison of staging by CEMDCT and PET/CT with histopathologic TNM staging revealed a higher accuracy of primary tumor staging with PET/CT while the accuracy for detection of distant metastasis was same with both the modalities. The accuracy for regional lymph node staging was poor with both CEMDCT and PET/CT although it was better with CEMDCT. Hence, we feel that in view of higher accuracy of primary tumor staging, PET/CT can be considered as a first line diagnostic modality for the preoperative evaluation of patients with known, or strongly suspected colorectal carcinoma.
References
- Boyle P, Leon ME (2002) Epidemiology of colorectal cancer. Br Med Bull 64:1-25.
- Mohandas KM, Desai DC (1999) Epidemiology of digestive tract cancers in India. V. large and small bowel. Indian J Gastroenterol18:118-121.
- Zerhouni EA, Rutter C, Hamilton SR, Balfe DM, Megibow AJ, et al. (1996) CT and MR imaging in the staging of colorectal carcinoma: Report of the Radiology Diagnostic Oncology Group II. Radiology 200:443-451.
- Delbeke D, Vitola JV, Sandler MP, Arildsen RC, Powers TA, et al. (1997)Staging recurrent metastatic colorectal carcinoma with PET. J Nucl Med 38:1196-1201.
- Macdonald JS (1999) Adjuvant therapy of colon cancer. CA Cancer J Clin49:202-219.
- Compton CC, Greene FL (2004)The Staging of Colorectal Cancer: 2004 and Beyond. CA Cancer J Clin 54:295-308.
- Thoeni RF, Moss AA, Schnyder P, Margulis AR (1981) Detection and staging of primary rectal and rectosigmoid cancer by computed tomography. Radiology 141:135-138.
- Balthazar EJ, MegibowAJ, Hulnick D, Naidich DP (1998) Carcinoma colon: detection and preoperative staging by CT. Am J Roentgenol 150:301-306.
- Acunas B, Rozanes I, Acunas G, Celik L, Sayi I, et al. (1990)Preoperative CT staging of colon carcinoma (excluding the rectosigmoid region). Eur J Radiol 11:150-153.
- Angelelli G, Macarini L, Lupo L, Caputi-Jambrenghi O, Pannarale O, et al. (1990) Rectal carcinoma: CT staging with water as contrast medium. Radiology 177:511-514.
- Chiesura-Corona M, Muzzio PC, Giust G, Zulliani M, Pucciarelli S, et al. (2001)Rectal cancer: CT local staging with histopathologic correlation. Abdom Imaging 26:134-138.
- Iannaccone R, Laghi A, PassarielloR (2005)Colorectal carcinoma: detection and staging with multislice CT (MSCT) colonography. Abdom Imaging 30:13-19.
- Shin SS, Jeong YY, Min JJ, Kim HR, Chung TW, et al. (2008) Preoperative staging of colorectal cancer: CT vs. integrated FDG PET/CT Abdom Imaging 33:270-277.
- Dirisamer A, Halpern BS, Flory D, Wolf F, Beheshti M, et al. (2010) Performance of integrated FDG-PET/contrast-enhanced CT in the staging and restaging of colorectal cancer: Comparison with PET and enhanced CT. Eur J Radiol 73:324-328.
- Mainenti PP, Iodice D, Segreto S, Storto G, Magliulo M, et al. (2011)Colorectal cancer and 18FDG-PET/CT: What about adding the T to the N parameter in loco-regional staging? World J Gastroenterol 17:1427-1433.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences