Immunohistochemical Localization of KRAS and BRAF and its Clinical Utility in Patients with Colorectal Cancer
Kanik P, Gajjar K and Ghosh N*
DOI10.21767/2471-9943.100051
Kanik P1, Gajjar K2 and Ghosh N2*
1Biochemistry, St. Xavier’s College (Autonomous), Ahmedabad, Gujarat, India
2Tumor Biology Lab 2, Cancer Biology Department, The Gujarat Cancer and Research Institute, Asarwa, Ahmedabad, Gujarat, India
- *Corresponding Author:
- Dr. Nandita Ghosh
Assistant Professor and Head
Tumor Biology Lab 2, Cancer Biology Department
The Gujarat Cancer and Research Institute
NCH Compound, Asarwa, Ahmedabad-380016, India
Tel: 07922688244
E-mail: nandita.ghosh@gcriindia.org
Received date: June 01, 2018; Accepted date: June 22, 2018; Published date: June 26, 2018
Citation: Kanik P, Gajjar K and Ghosh N (2018) Immunohistochemical Localization of KRAS and BRAF and its Clinical Utility in Patients with Colorectal Cancer. Colorec Cancer. Vol.4 No.1:04. doi:10.21767/2471-9943.100051
Copyright: © 2018 Kanik P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Colorectal cancer (CRC) is a multistep process occurring due to the accumulation of several genetic alterations. The most important ones are RAS and RAF that have been implicated as key intermediates in RAS-mediated signaling cascade. KRAS and BRAF protein expression levels and prognostic evaluation in CRC patients remains unknown. Moreover, minimal evidence exists regarding the predictive role of KRAS and BRAF for fluoropyrimidine-based adjuvant chemotherapy. Hence, present study aimed to investigate the prevalence of KRAS and BRAF protein expressions in CRC patients and further to correlate the results with clinicopathological parameters and prognosis.
Methods and findings: A total 82 CRC patients were included in this retrospective study. KRAS and BRAF protein expressions were studied by immunohistochemistry technique using paraffin embedded tumor tissue blocks. The data was analyzed using SPSS software. p value = 0.05 was considered as statistically significant. Immunohistochemical localization of KRAS and BRAF showed cytoplasmic staining in 34% and 63% of patients, respectively. BRAF immunopositivity was found to be significantly higher in patients with positive nodal status (p=0.018), presence of perineural invasion (p=0.039) and = 5.0 ng/mL pre-operative serum CEA levels (p=0.008) as compared to their respective counterparts. Moreover, a trend of higher positive BRAF expression was observed in advanced stage patients as compared to early stage patients (p=0.080). Survival analysis demonstrated a significant reduced relapse-free survival (RFS) with KRAS positive expression in rectal cancer patients (p=0.045). Similar trend of reduced RFS was observed with positive KRAS expression in the subgroup of rectal cancer patients treated with adjuvant therapy (p=0.073). However, BRAF protein expression failed to show any prognostic or predictive value in CRC patients.
Conclusion: KRAS and BRAF protein expression might be associated with disease aggressiveness in CRC. Further, KRAS protein expression could be useful prognostic and predictive marker in rectal cancer patients.
Keywords
KRAS; BRAF; Immunohistochemistry; Prognosis; Colorectal cancer
Abbreviations
BRAF: v-Raf murine sarcoma viral oncogene homolog B1; CEA: Carcino Embryonic Antigen; CRC: Colorectal Cancer; EGFR: Epidermal Growth Factor Receptor; IHC: Immunohistochemistry; KRAS: v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog; MAPK: Mitogen-Activated Protein Kinase; OS: Overall Survival; PTC: Papillary Thyroid Cancer; RFS: Relapse-Free Survival; SPSS: Statistical Package for the Social Sciences.
Introduction
Colorectal cancer (CRC) is a heterogeneous disease that manifests through variable clinical course and with significant inconsistency in the response to treatment, even within tumors with similar histopathological characteristics [1]. Current researchers mostly focus on the theory of heterogeneity of CRC tumors, which highlights the differences of the KRAS mutational status between primary and metastatic tumors [2]. Moreover, abnormalities of KRAS are key events in colorectal carcinogenesis and mutations in this gene occur early during malignant transformation in 20-50% of the tumors [3]. KRAS is a major downstream component of the epidermal growth factor receptor (EGFR) pathway [4], and it activates its primary downstream target protein BRAF, a serine-threonine protein kinase that mediates the KRAS signal toward the downstream effectors, eventually leads to the activation of genes involved in cell proliferation. The KRAS and BRAF mutations as key mediators in EGFR signaling pathway play a crucial role in the colorectal pathogenesis and are associated with primary resistance to EGFR therapy [2].
Although the predictive role of KRAS and BRAF mutations to recognize resistance to anti-EGFR therapy in advanced CRC patients has been accepted widely, the efficacy of KRAS and BRAF protein expressions in CRC is still unidentified. Several reports investigated KRAS immunohistochemical localization, but it requires further studies for validation as a predictive marker for resistance to anti-EGFR therapy [5]. Moreover, minimal evidence exists regarding the predictive capacity of KRAS with fluoropyrimidine-based adjuvant chemotherapy [6]. Further, KRAS expression levels and prognostic evaluation in CRC patients remains unknown. On the other side, clinical studies have shown an association between BRAF mutation and clinical progression, invasion, and recurrence in various malignancies including CRC. However, there are rare studies showing BRAF protein expression especially in thyroid carcinoma [7,8]. To the best of our knowledge, there are no studies available regarding investigations of BRAF protein expression in CRC. Therefore, exploring KRAS and BRAF protein expression could be helpful in management of CRC patients.
In the present study, we investigated the incidence of KRAS and BRAF protein expression and their association with clinicopathological parameters in CRC patients. In addition, the prognostic and predictive role of KRAS and BRAF protein expression was analyzed in CRC.
Materials and Methods
Patients
A total of 82 CRC patients underwent surgical resection at The Gujarat Cancer and Research Institute, Ahmedabad between 2013 and 2015 were included in this study. The key inclusion criterion was the untreated CRC patients with histopathologically confirmed adenocarcinoma without any prior history of anticancer treatment. Patient and tumor characteristics are shown in Table 1. The primary treatment offered to the patients was either surgery alone (20%, 16/82) or surgery followed by chemotherapy (56%, 46/82) or surgery followed by chemoradiotherapy (19%, 16/82) or surgery followed by radiotherapy (5%, 4/82). The main chemotherapeutic treatment included were 5-fluorouracil, capecitabine or in combination with oxaliplatin. Out of 82 patients enrolled, 78 patients who followed up for the period of 24 months or until death within that period, were included for Overall survival (OS) analysis.
| Characteristics | N (%) |
| Age (years) (Range: 20-80 years) Median age: 56 years | |
| <56 | 41 (50) |
| = 56 | 41 (50) |
| Gender | |
| Female | 37 (45) |
| Male | 45 (55) |
| Habit* | |
| No | 44 (54) |
| Yes | 38 (46) |
| Tumor site | |
| Colon | 48 (59) |
| Rectum | 34 (41) |
| Tumor size | |
| T2 | 14 (17) |
| T3 | 62 (76) |
| T4 | 06 (07) |
| TNM stage | |
| I | 08 (10) |
| II | 39 (48) |
| III | 32 (39) |
| IV | 03 (03) |
| Early stage (I+II) | 47 (57) |
| Advanced stage (III+IV) | 35 (43) |
| Histological type | |
| Adenocarcinoma | 61 (74) |
| Mucinous adenocarcinoma | 21 (26) |
| Tumor differentiation | |
| Well | 13 (16) |
| Moderate | 59 (72) |
| Poor | 10 (12) |
| Recurrence/Metastasis (N=63) | |
| Absent | 57 (90) |
| Present | 06 (10) |
| Disease outcome (N=78) | |
| Alive | 62 (79) |
| Dead | 16 (21) |
Note: *Tobacco chewing, smoking, alcohol, snuff (any one or in combination).
Table 1: Patient and tumor characteristics. *Tobacco chewing, smoking, alcohol, snuff (any one or in combination).
For OS, three patients who died with advanced stage disease and one patient who lost to follow-up were excluded. Out of these 78 patients, 15 patients with persistent disease were excluded from relapse-free survival (RFS) analysis. Thus, 63 patients were included for RFS analysis. Survival analysis was also performed in the subgroups of patients with early stage and advanced stage disease; as well as in colon cancer and rectal cancer patients after sub-grouping them according to tumor site. Further, to investigate the predictive value of KRAS and BRAF, survival analysis was performed in patients treated with adjuvant therapy (RFS: N=54; OS: N=64).
Sample collection
The study has been approved by Institutional Scientific and Ethical Committees and informed consent was obtained from all patients prior to sample collection. To study KRAS and BRAF protein expression, paraffin embedded tumor tissue blocks were retrieved from histopathology department of the institute.
KRAS and BRAF protein expression by immunohistochemistry
Immunohistochemistry for KRAS and BRAF was performed on formalin-fixed, paraffin embedded 4 μm thick tumor tissue sections. The sections were stained using mouse and rabbit specific HRP/DAB (ABC) Detection IHC kit (Abcam) according to manufacturer’s protocol. The primary antibody used was KRAS mouse monoclonal (Clone-F234, Santacruz biotechnology dilution: 1:10) and BRAF mouse monoclonal (Clone-1H12F1, 1H12G10, 1F12F11C9, Thermo scientific, dilution: 1:200). Prior to application of the primary antibody, antigen retrieval was performed for 20 minutes in a pressure cooker. All sections were scored in a blinded fashion by two independent observers familiar with immunohistopathology, unaware of clinical outcome of the patient. A semiquantitative score ranging from negative (no staining or, 10% of cells stained) to 3+ (1+ staining in 11-30% of the cells: weak, 2+ staining in 31-50% of cells: moderate, and 3+ staining in >50% of cells: intense) was used.
Statistical analysis
The statistical data analysis was performed with the help of SPSS (Statistical Package for the Social Sciences) software version 17. Two tailed chi square (χ2) test was used to determine the association of clinicopathological variables with KRAS and BRAF protein expression. Correlation between two parameters was calculated using Spearman’s correlation coefficient (r) method. RFS and OS were calculated using Kaplan-Meier estimates and the difference in survival curve was calculated using Log rank test. P value ≤ 0.05 was considered to be significant.
Results
Incidence of KRAS and BRAF protein expression
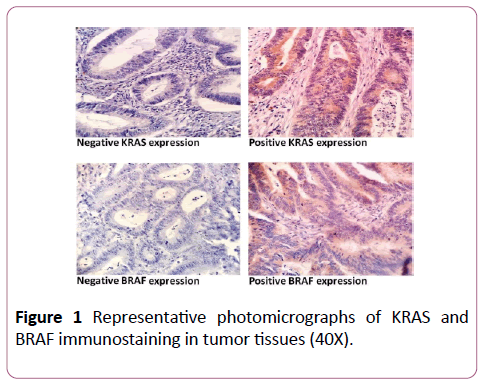
Both KRAS and BRAF protein expression showed heterogeneous and cytoplasmic immunostaining pattern. KRAS immunopositivity was detected in 34% of tumors, while 66% of tumors were KRAS negative. On the other side, 63% of tumors showed BRAF positive protein expression, while 37% of tumors were BRAF negative. Figure 1 represents the photomicrographs for KRAS and BRAF staining (40x).
Association of KRAS and BRAF protein expression with clinicopathological parameters
BRAF immunopositivity was found to be significantly higher in patients with positive nodal status (79%) as compared to those with negative nodal status (54%; χ2=5.609, r=+0.262, p=0.018); in patients with presence of perineural invasion (87%) as compared to those with absence of perineural invasion (58%, χ2=4.278, r=+0.228, p=0.039); as well as in patients with ≥ 5.0 ng/ mL pre-operative serum CEA levels (76%) as compared to those with <5.0 ng/mL pre-operative CEA levels (47%; χ2=6.885, r=+0.292, p=0.008). Further, a trend of higher positive BRAF protein expression was found in advanced stage (III+IV) patients (74%) as compared to early stage (I+II) patients (55%; χ2=3.111, r=+0.195, p=0.080). However, KRAS protein expression showed no significant correlation with any of the clinicopathological parameters.
Association of KRAS and BRAF protein expression with survival
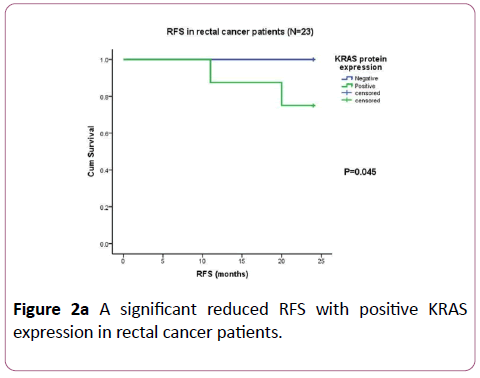
When prognostic value of KRAS protein expression was evaluated, Kaplan-Meier univariate survival analysis showed a significant association of KRAS protein expression with RFS in the subgroup of rectal cancer patients only (N=23). A significant higher incidence of disease relapse was observed in rectal cancer patients with positive KRAS protein expression (25%) as compared to those with negative KRAS protein expression (0%; χ2=0.4010, df=1, p=0.045; Figure 2a).
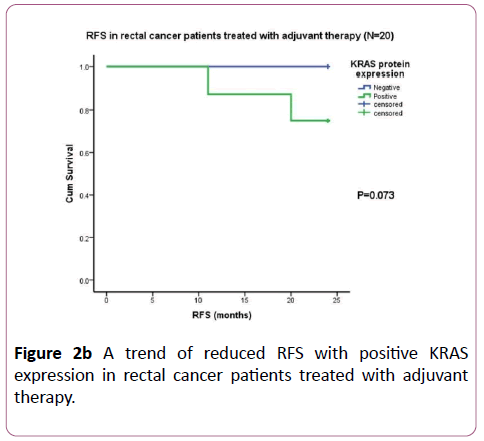
However, no significant association of KRAS protein expression with RFS or OS was observed in total patients or in the subgroups of early stage, advanced stage and colon cancer patients (data not shown). Further, when predictive value of KRAS protein expression was evaluated in patients treated with adjuvant therapy (N=54), only in the subgroup of rectal cancer patients (N=20), KRAS protein expression showed a trend of higher incidence of disease relapse in patients with positive KRAS expression (25%) as compared to those with negative KRAS expression (0%; χ2=3.209, df=1, p=0.073; Figure 2b). On the other side, BRAF protein expression failed to show any prognostic or predictive value when correlated with survival (data not shown).
Correlation between KRAS and BRAF protein expression
Positive correlation was observed between KRAS and BRAF protein expression. However, the data was not statistically significant (r=+0.120, p=0.284).
Discussion
The development of colorectal cancer is a multistep process that occurs due to the accumulation of several genetic alterations. The most important ones are RAS and RAF that have been implicated as key intermediates in RAS-mediated signaling cascade [9].
RAS proteins are proto-oncogenes that are frequently mutated in human cancers. Despite the prevalent role of KRAS mutations in tumorigenesis, few studies have been reported with regard to KRAS protein expression, especially in CRC. Several recent studies indicated that KRAS amplification occurred only in a small percentage of several different solid malignancies, including non small cell lung cancer, head and neck squamous cell carcinoma, ovarian and breast cancer [5]. One report in CRC showed that KRAS amplification was an infrequent event (0.67%) and found to be mutually exclusive with KRAS mutations. They further compared KRAS gene copy numbers by real-time PCR with IHC KRAS status using the same clone SC-30, which we used in present study, and found that all positive cases for real-time PCR were strongly positive by IHC technique also. But it requires further studies for validation as a predictive marker for resistance to anti-EGFR therapy [5]. Further, Horsch et al. showed genome-wide transcriptional changes in KRAS transfected cell lines [10]. More recently, Zhou et al. focused on RAS expressions by realtime PCR in AML and detected KRAS overexpression in 35/143 (24%) of AML patients [11].
Present study examined KRAS protein expression by IHC in CRC and observed cytoplasmic KRAS immunopositivity in 34% of tumors. Further, none of the clinicopathological parameters were found to be significantly correlated with KRAS protein expression. Likewise, Piton et al. in patients with CRC observed distinct cytoplasmic staining of KRAS expression in neoplastic areas in 33% of patients [12]. A small number of studies have examined IHC localization of KRAS and its association with clinicopathological parameters in CRC. In accordance with present study, one report in metastatic CRC observed KRAS cytoplasmic positivity in 42.3% of cases and found no significant correlation between clinicopathological parameters and KRAS staining results [13]. Another study conducted by Zlatian et al. in 50 CRC patients detected KRAS immunopositivity in 52% of cases and observed that KRAS expression was significantly increased in poorly differentiated adenocarcinoma (p=0.037) [14].
KRAS expression levels and prognostic evaluation in CRC patients remains unknown. To the best of our knowledge, this is the first study to demonstrate prognostic and predictive role of KRAS immunohistochemical localization in CRC. We observed that the subgroup of rectal cancer patients showed a significant reduced RFS with KRAS positive expression as compared to KRAS negative expression (p=0.045). Similar trend of reduced RFS was observed with positive KRAS expression in the subgroup of rectal cancer patients treated with adjuvant therapy (p=0.073).
Hence, present study demonstrated that positive cytoplasmic KRAS protein expression probably could be a useful biomarker in predicting unfavorable prognosis and treatment response in rectal cancer patients. However, further studies with larger patient size and longer follow-up are needed to better understand the prognostic and predictive role of KRAS protein expression in CRC, which would aid in determination of therapeutic response and prognosis of patients.
BRAF, the downstream target of KRAS, is a key activator of the MAPK pathway that transmits signals from the surface of cells to the nucleus and regulates cell survival, differentiation, apoptosis and proliferation. When it is activated by mutation, inappropriate signaling promotes malignant cell proliferation and survival. BRAF protein expresses with varied levels according to the type of tissue. Several recent studies have been aimed to determine the sensitivity, specificity and predictive values of specific monoclonal antibody (VE1 clone) which targets the mutated form of the Braf protein (V600E Braf) since immunohistochemical testing would be an optimal, an inexpensive and widely available clinically applicable methodology of testing for the Braf V600E mutation in routine practice.
In the present study, BRAF (Clone 1H12F1, 1H12G10, 1F12F11C9) protein expression showed heterogeneous and cytoplasmic staining pattern. Positive immunoreactivity was noted in 63% of CRC patients, while 37% of CRC patients were BRAF negative. In accordance to the present study, Piton et al. in patients with CRC observed finely granular cytoplasmic staining of BRAF (VE1) expression in neoplastic areas in 66% patients [12]. Affolter et al. analyzed BRAF specific to the V600E-mutated protein in 31 colon tumors with (n=14) and without (n=17) the BRAF V600E mutation [15]. They demonstrated cytoplasmic positivity in tumor cells in 45% (14/31) tumors. Sinicrope et al. [16] in their study used two different types of BRAF antibody (pan-BRAF antibody and BRAF V600E) in human colon carcinomas with the aim to evaluate an anti-BRAF antibody for the detection of mutant BRAF V600E proteins. Using a pan-BRAF antibody, total protein expression was observed in the tumor cell cytoplasm in 74 of 75 colon carcinomas whereas BRAF V600E antibody identified diffuse cytoplasmic staining in 49 of 74 (66%) cancers. In contrast, comparably, lower incidence of BRAF positivity was reported by Capper et al. [17] and Day et al. [18]. In both the studies, the authors used BRAF (VE1) antibody and observed finely granular cytoplasmic staining reaction in tumor cells in only 13.2% of colorectal cancer specimens. Beside CRC, there are reports on BRAF protein expression in other malignancies. Mohamad et al. examined the impact of BRAF protein in 60 Iraqi breast cancer patients and observed BRAF was positive in 28.57% of benign sections and 65.96% of malignant sections while totally negative expression in normal breast tissue sections [19]. Jung et al. reported cytoplasmic and nuclear protein expression of BRAF in 13% and 7.4% of breast cancer patients, respectively using BRAF V600E mutation specific antibody [20]. Feng et al. [8] and da Silva et al. [7] observed BRAF (F-7) cytoplasmic immunoreactivity in 60% and 57% of patients with thyroid cancer, respectively. In melanoma, Hugdahl et al. [21] and Elmageed et al. [22] reported positive cytoplasmic BRAF-V600E expression in 35% and 32% of the cases, respectively. Further, Elmageed et al. observed both cytoplasmic and nuclear staining pattern of BRAF-V600E in 30% cases [22]. Wen et al. studied the expression and clinical significance of BRAF in prostate cancer; they found the positive rate of BRAF had significant difference between patients with prostate cancer and BPH (p<0.05) [23].
It is well established that expression of the BRAF protein is of important clinical interest, particularly in the case of resistance to conventional therapy. To the best of our knowledge, less information is available on BRAF protein expression with regard to clinicopathological variables in CRC. Capper et al. observed CRC patients with BRAF-positive tumors were of older age and female patients and no significant difference was found of BRAF positivity according to tumor site [17]. Further, Day et al. reported a higher frequency of BRAF V600E positivity in female patients and tumors resected from the right colon, exhibiting poor differentiation [18]. Present study too speculated a significant higher BRAF immunopositivity in patients with positive lymph-nodes (79%), with presence of perineural invasion (87%); as well as in patients with ≥ 5.0 CEA levels (76%) as compared to their respective counterparts. Furthermore, a trend of higher positive BRAF protein expression was found in advanced stage patients (74%) as compared to early stage patients (55%). Further, higher positive BRAF protein expression was observed in T4 tumor (83%) than those with T2 (64%) and T3 tumor (61%) size; and in elder age group (71%) than in younger age group patients (56%), however such associations were not statistically significant. In accordance to the present study, besides CRC, in breast cancer patients, Mohamad et al. reported that BRAF status had significant association with increasing age and tumor stage and no significant correlation with tumor grade [19]. Jung et al. observed that nuclear but not cytoplasmic BRAF V600E status was significantly associated with poor prognostic factors such as ER negativity, PR negativity and TNBC molecular subtype [20]. Similarly, Feng et al. [8] highlighted significant association of BRAF over expression with worse prognostic factors such as lymph node metastatic group than in the non- lymph node metastatic group; and in papillary thyroid cancer than in nodular goiter patients. Likewise, da Silva et al. found significant association of BRAF over expression and extrathyroidal extension of the tumor which represents a worse prognosis [7]. Wen et al. in patients with prostate cancer found that the expression of BRAF was correlated with grade and stage of prostate cancer (P<0.05), but not to age of onset (P>0.05) [23]. Elmageed et al. reported that nuclear localization of BRAF V600E was significantly associated with melanoma aggressiveness viz overall clinical stage, regional lymph node, depth of invasion, Clark level, mitotic activity, ulceration [22]. In accordance to above reports, our study demonstrated that BRAF over expression in the patients with CRC was indeed associated with worse prognostic factors. Contradictory, Koperek et al. showed that the expression of the mutated BRAF V600E protein seems not to be a marker of aggressiveness but is already seen in clinically indolent microcarcinomas [24].
With regard to prognosis, in the current study presence or absences of BRAF protein expression had no impact on survival in CRC patients. In contrast, Day et al. reported significant higher risk with poor OS for metastatic CRC patients with BRAF V600E tumors on IHC as compared to those with BRAF wild type [18]. Hugdahl et al. reported BRAF-V600E protein expression detected by IHC is independently associated with reduced survival, whereas this was not the case for BRAF mutation status [21]. Literature survey revealed several conflicting reports on influence of BRAF mutation on survival in various cancers including CRC. However, there exists scares report on BRAF protein expression and survival. Further, present study investigated intercorrelation between KRAS protein expression and BRAF protein expression. However, no significant correlation was observed between them. Therefore, further studies are required for the better evaluation of BRAF protein expression and its correlation with KRAS protein expression in CRC.
Conclusion
Present study concluded that KRAS and BRAF positive protein expression might be associated with disease aggressiveness in CRC. Moreover, KRAS protein expression might be helpful to predict prognosis and treatment response and thus could be useful prognostic and predictive marker in rectal cancer patients. Our findings may contribute into evolution of better therapeutic approach in CRC patients. However, this data needs to be validated in larger cohort to potentially influence treatment decisions in CRC.
Funding
This study was financially supported by The Gujarat Cancer and Research Institute/ Gujarat Cancer Society.
Conflict of Interest
None.
References
- Blanco-CM, Concha A, Figueroa A, Garrido F, Valladares AM (2015) Colorectal cancer classification and cell heterogeneity: a systems oncology approach. Int J Mol Sci 16: 13610-13632.
- Larki P, Gharib E, Taleghani MY, Khorshidi F, Nazemalhosseini ME, et al. (2017) Coexistence of KRAS and BRAF Mutations in Colorectal Cancer: A case report supporting the concept of tumoral heterogeneity. Cell J 19: 113-117.
- Miranda E, Destro A, Malesci A, Balladore E, Bianchi P, et al. (2006) Genetic and epigenetic changes in primary metastatic and nonmetastatic colorectal cancer. Br J Cancer 95: 1101-1107.
- Won DD, Lee JI, Lee IK, Oh ST, Jung ES, et al. (2017) The prognostic significance of KRAS and BRAF mutation status in Korean colorectal cancer patients. BMC cancer 17: 403.
- Valtorta E, Misale S, Sartore BA, Nagtegaal ID, Paraf F, et al. (2013) KRAS gene amplification in colorectal cancer and impact on response to EGFR-targeted therapy. Int J Cancer 133: 1259-1265.
- Hutchins G, Southward K, Handley K, Magill L, Beaumont C, et al. (2011) Value of mismatch repair, KRAS, and BRAF mutations in predicting recurrence and benefits from chemotherapy in colorectal cancer. J Clin Oncol 29: 1261-1270.
- da Silva RC, De Paula HS, Leal CB, Cunha BC, De Paula EC, et al. (2015) BRAF overexpression is associated with BRAF V600E mutation in papillary thyroid carcinomas. Genet Mol Res 14: 5065-75.
- Feng L, Li M, Zhang QP, Piao ZA, Wang ZH, et al. (2011) Utility of BRAF protein overexpression in predicting the metastasis potential of papillary thyroid carcinoma. Oncology letters 2: 59-63.
- Calabretta B, Kaczmarek L, Ming PM, Au F, Ming SC (1985) Expression of c-myc and other cell cycle-dependent genes in human colon neoplasia. Cancer Research 45: 6000-6004.
- Horsch M, Recktenwald CV, Schadler S, De Angelis MH, Seliger B, et al. (2009) Overexpressed vs mutated Kras in murine fibroblasts: a molecular phenotyping study. Br J Cancer 100: 656-662.
- Zhou JD, Yao DM, Li XX, Zhang TJ, Zhang W, et al. (2017) KRAS overexpression independent of RAS mutations confers an adverse prognosis in cytogenetically normal acute myeloid leukemia. Oncotarget 8: 66087-66097.
- Piton N, Borrini F, Bolognese A, Lamy A, Sabourin JC (2015) KRAS and BRAF mutation detection: is immunohistochemistry a possible alternative to molecular biology in colorectal cancer? Gastroenterol Res Pract 2015: 753903.
- Elsabah MT, Adel I (2013) Immunohistochemical assay for detection of K-ras protein expression in metastatic colorectal cancer. J Egypt Natl Canc Inst 25: 51-56.
- Zlatian OM, Comanescu MV, Rosu AF, Rosu LU, Cruce M, et al. (2015) Histochemical and immunohistochemical evidence of tumor heterogeneity in colorectal cancer. Rom J Morphol Embryol 56: 175-181.
- Affolter K, Samowitz W, Tripp S, Bronner MP (2013) BRAF V600E mutation detection by immunohistochemistry in colorectal carcinoma. Genes Chromosomes Cancer 52: 748-752.
- Sinicrope FA, Smyrk TC, Tougeron D, Thibodeau SN, Singh S, et al. (2013) Mutation-specific antibody detects mutant BRAFV600E protein expression in human colon carcinomas. Cancer 119: 2765-2770.
- Capper D, Voigt A, Bozukova G, Ahadova A, Kickingereder P, et al. (2013) BRAF V600E-specific immunohistochemistry for the exclusion of Lynch syndrome in MSI-H colorectal cancer. Int J Cancer 133: 1624-1630.
- Day F, Muranyi A, Singh S, Shanmugam K, Williams D, et al. (2015) A mutant BRAF V600E-specific immunohistochemical assay: correlation with molecular mutation status and clinical outcome in colorectal cancer. Target oncol 10: 99-109.
- Mohamed Suhaimi NA, Foong YM, Lee DY, Phyo WM, Cima I, et al. (2015) Non-invasive sensitive detection of KRAS and BRAF mutation in circulating tumor cells of colorectal cancer patients. Mol Oncol 9: 850-860.
- Jung YY, Jung WH, Koo JS (2016) BRAF mutation in breast cancer by BRAF V600E mutation-specific antibody. Int J Clin Exp Pathol 9: 1545-1556.
- Hugdahl E, Kalvenes MB, Puntervoll HE, Ladstein RG, Akslen LA (2016) BRAF-V600E expression in primary nodular melanoma is associated with aggressive tumour features and reduced survival. Br J Cancer 114: 801-808.
- Elmageed ZY, Moore RF, Tsumagari K, Lee MM, Sholl AB, et al. (2018) Prognostic Role of BRAFV600E Cellular Localization in Melanoma. J Am Coll Surg 226: 526-537.
- Wen Z, Zou X, Yang G, Wang H (2011) The expression and clinical significance of BRAF in prostate cancer. The Chinese-German Journal of Clinical Oncology 10: 523.
- Koperek O, Kornauth C, Capper D, Berghoff AS, Asari R, et al. (2012) Immunohistochemical detection of the BRAF V600E-mutated protein in papillary thyroid carcinoma. The American Journal of Surgical Pathology 36: 844-850.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences