Comparison of Genetic, Protein Expression and Clinicopathological Parameters in Patients with Colorectal Cancer having Vegetarian vs Mixed Diet
Nikhil Garg
Department of Surgical Oncology, University of AIIMS, Bathinda, India
Published Date: 2023-04-24DOI10.36648/2471-9943.9.1.03
Chamanjot Singh1 and Nikhil Garg2*
1Department of Surgery, Civil Hospital, Malerkotla, India
2Department of Surgical Oncology, University of AIIMS, Bathinda, India
- *Corresponding Author:
- Nikhil Garg
Department of Surgical Oncology,
University of AIIMS, Bathinda,
India,
E-mail: nikhilgarg9490@ gmail.com
Received date: March 23, 2023, Manuscript No. IPJCC-23-16142; Editor assigned date: March 27, 2023, PreQC No. IPJCC-23-16142 (PQ); Reviewed date: April 06, 2023, QC No. IPJCC-23-16142; Revised date: April 17, 2023, Manuscript No. IPJCC-23-16142 (R); Published date: April 24, 2023, DOI: 10.36648/2471-9943.9.1.03.
Citation: Singh C, Garg N (2023) Comparison of Genetic, Protein Expression and Clinicopathological Parameters in Patients With Colorectal Cancer Having Vegetarian vs. Mixed Diet. Colorec Cancer Vol.9 No.1: 03.
Abstract
Purpose: To assess the prevalence of KRAS codon 12/13 mutations and protein expression in patients with Colorectal Cancer (CRC). The outcomes were correlated with clinicopathological characteristics in vegetarian versus mixed diet population.
Methods: Dietary patterns were correlated with demographic and clinicopathological characteristics including KRAS codon 12/13 mutations and their protein expressions.
Results: Retrospective analysis was performed on 78 CRC patients, 55% of whom were men and had a median age of 56 years. Majority of the patients were vegetarian (73%) while remaining had a mixed diet. The association between dietary patterns and clinicopathological characteristics was observed and vegetarianism was more prevalent in patient with early and advanced cancer stages. The final histopathology revealed a statistically significant correlation between KRAS codon 12 mutations (23%) and necrosis (p=0.025) as well as a higher death rate (p=0.021). In the subgroup of rectal cancer patients, positive K-ras protein expression was linked to a higher risk of disease relapse (p =0.045). Vegetarians had higher levels of B-raf protein expression and was associated with poor prognostic factors, such as perineural invasion (86%) (p=0.051), positive lymph nodes (p=0.020) and high circulating carcinoembryonic antigen levels (>5.0 ng/ml) (p=0.006).
Conclusion: In our study, we found a higher prevalence of vegetarianism in patients with both early and advanced cancer stages. Among vegetarian patients, a link between B-raf protein expression with unfavorable prognostic factors was also observed. KRAS codon 12/13 mutations and K-ras/ B-raf protein expression may act as vital prognostic and predictive indicators in CRC patients.
Keywords
Colorectal cancer; BRAF; KRAS; Dietary pattern
Introduction
Recent advancements in our understanding of the pathophysiology of Colorectal Cancer (CRC) have led to increased therapeutic options. They include endoscopic and surgical local excision, down staging pre-operative radiotherapy and systemic therapy, extensive surgery for locoregional and metastatic disease, local ablative therapies for metastases, palliative chemotherapy, targeted therapy and immunotherapy [1]. These treatments effectively and gradually reduce cancer progression and improve overall survival [2]. Additionally, proper enforcement of screening methods including fecal occult blood testing and endoscopy increases the survival rate and likelihood of CRC cure [3].
In 2020, GLOBOCAN estimated over 1.9 million new CRC cases with 0.93 million fatalities in 185 countries. The incidence and mortality rates were highest for males in European nations and lowest for females in African and South Asian nations. By 2040, it is estimated that 3.2 million new cases and 1.6 million deaths would occur from CRC. In developing countries such as India, CRC incidences were reported in a total of 0.065 million cases comprising 61.83% males and 38.17% females [4].
The rising incidence of CRC is mainly attributed to changes in lifestyle, aging and genetic aberrations. Numerous other risk factors, including poor diet, physical inactivity, smoking, chronic inflammatory bowel disease, advancing age and accumulation of genetic aberrations, have a substantial impact on the incidence and mortality of CRC [5]. Diet can influence the risk of CRC either through dietary components or indirectly through changes in gut flora or body weight. Healthy dietary patterns include high consumption of insoluble fiber, vegetables, fruits and low-fat milk. High consumption of processed foods, red meat, refined carbohydrates, high calories and a low-calcium diet promotes an inflammatory response and increases the risk of CRC [6]. Individuals who have a sedentary lifestyle or are obese have a 25%-50% higher risk of developing colon cancer compared to those who are physically active [7].
In addition to lifestyle factors, multiple genetic pathways and mechanisms contribute to CRC pathogenesis. The two important oncogenes that promote the progression of CRC are KRAS and BRAF, which are key players in the RAS/RAF/MEK/MAP kinase signaling pathways [8]. K-ras is a small GTPase transducer protein with intrinsic GTPase activity that controls regulatory pathways and promotes cell growth. B-raf is a serine-threonine protein kinase that acts as a direct effector of Ras and promotes tumor growth and survival by activating the Mitogen-Activated Protein Kinase (MAPK) pathway. It has been established that KRAS and BRAF mutations are present in approximately 40% and 10% of CRC cases, respectively [9,10]. The most frequent mutation in KRAS is an amino acid change at codon 12 or 13 in exon 2, whereas the most prevalent mutation in BRAF, V600E, occurs at codon 600 in exon 15 and accounts for 5%–10% of all cases of colon cancer [11].
Limited studies have examined genome-wide transcriptional alterations in KRAS and BRAF-transfected cell lines, despite their common significance in carcinogenesis [12]. Both dietary and genetic modifications play a significant role in the pathogenesis of CRC. Therefore, exploring the correlation between dietary patterns and K-ras and B-raf protein expression may be beneficial for the management of patients with CRC. In light of these factors, the objective of the present study was to investigate the prevalence of KRAS codon 12 and 13 mutations along with K-ras and B-raf protein expression in CRC patients to further correlate the findings with various clinicopathological parameters and to compare them with either vegetarian or mixed diets.
Materials and Methods
Patients
A total of 78 untreated and histologically confirmed colorectal cancer patients registered at ‘The Gujarat Cancer and Research Institute (GCRI)’, Ahmedabad, India, from 2013 to 2015 were enrolled in this study. The tissue acquired during the surgery was stored in the immunochemistry and cancer biology laboratory. The tests for KRAS 12/13 codons and K-ras and B-raf proteins were performed in 2017. The demographic characteristics of the patients including age, sex, family history, dietary habits, alcohol consumption, tobacco smoking and other potential confounding factors were collected. Detailed clinical and pathological characteristics of the patients including tumor size, lymph node status, histological grade, disease stage and treatment given, etc. were captured from the case files maintained at the medical record department of the institute and recorded in the registers kept at the tumor biology, GCRI. The key inclusion criteria were as follows: Untreated patients with histopathologically confirmed adenocarcinoma; patients with pre-op colonoscopy and biopsy; CT scan/MRI; patients with pre-op Carcinoembryonic Antigen (CEA) levels; and patients who had not previously received anticancer treatment at GCRI or any other hospital. The exclusion criteria included patients who underwent treatment prior to surgery (including previous surgery, chemotherapy and radiation therapy), as well as those who tested positive for HBsAg, HIV and HCV.
Sample collection
Prior to sample collection, written informed consent was obtained from each patient. Primary tumor tissue samples from 78 CRC patients were obtained on ice right from the operation theater for the analysis of K-ras codon 12 and 13 mutations. The tumor tissue portion from the collected specimen was selected by a pathologist, which was then snap frozen in liquid nitrogen and preserved at -80°C. Paraffin-embedded tumor tissue blocks from all patients were retrieved from the histopathology department of the institute for the analysis of K-ras and B-raf protein expression.
Methodology
The dietary patterns of the patients were analyzed and correlations were made with different demographic and clinicopathological details including K-ras codon 12 and 13 mutations and expression of K-ras and B-raf proteins.
Statistical analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences software (SPSS). Relapse-free survival and overall survival were calculated using the Kaplan-Meier method and Log-rank tests. The correlation between the two parameters was calculated using Spearman’s correlation coefficient (r). P-value ≤ 0.05 was considered significant.
Results
Demographic characteristics
A retrospective analysis was performed on 78 CRC patients, comprising 55% males and 45% females, with a median age of 56 years (range 20-80 years). Demographic details are provided in Table 1. A total of 73% of the patients were vegetarian, while the remaining patients had a mixed diet including a nonvegetarian or eggetarian diet. In addition, 47% of patients were tobacco consumers, smokers and alcoholics. The majority (59%) of patients had tumors in the colon while the remaining (41%) had a tumor in the rectum. The patients were at different stages of cancer; 74% had T3 tumors, 18% had T2 tumors and 8% had T4 tumors. According to the AJCC clinical staging system, 47% and 41% of the patients had stage II and III tumors, respectively. The colorectal cancer staging criteria used by Duke identified, 59% of the patients had Duke B and 41% had Duke C. Patients with Duke D were excluded from the study. In 74% of the cases, a moderately differentiated tumor was present while a poorly differentiated tumor was found in 11% of cases. Histological examination revealed adenocarcinoma in 73% and mucinous adenocarcinoma in 27% of cases. Nodal status was negative in 63% of the patients, while 37% had nodal-positive disease. In the analysis of tumor characteristics, 91% of patients did not show necrosis. Lymphatic permeation was absent in 80% of patients. Vascular permeation and perineural invasion were absent in 95% and 82% of the patients, respectively. Preoperative CEA levels were ≥ 5 ng/ml in 55% of the cases and ≤ 5 ng/ml in 45% of the cases. In 18% of the cases, the only form of treatment was surgery, in 58% of cases chemotherapy was added to surgery and radiotherapy added to surgery in 19% of cases. All three modalities were used in 5% of cases. Of the 63 patients studied, 57 (90%) exhibited no recurrence, while six experienced recurrence. Overall Survival (OS) was observed in 80% of patients (n=62), whereas 20% of patients (n=16) died during the follow-up period.
| Characteristics | N=78 | Percentage (%) | |
|---|---|---|---|
| Age (Range: 20-80 years) | <56 | 34 | 44 |
| (Median age: 56 years) | >56 | 44 | 56 |
| Sex | Female | 35 | 45 |
| Male | 43 | 55 | |
| Diet | Vegetarian | 57 | 73 |
| Mixed | 21 | 27 | |
| Habit | No | 41 | 53 |
| Yes | 37 | 47 | |
| Tumor site | Colon | 46 | 59 |
| Rectum | 32 | 41 | |
| Tumor size | T2 | 14 | 18 |
| T3 | 58 | 74 | |
| T4 | 6 | 8 | |
| AJCC staging | I | 9 | 11 |
| II | 37 | 47 | |
| III | 32 | 41 | |
| Early stage | 46 | 59 | |
| (Stage I+Stage II) | 32 | 41 | |
| Advanced stage | 46 | 59 | |
| (Stage III+Stage IV) | 32 | 41 | |
| Dukes’ stage | B | 46 | 59 |
| C | 32 | 41 | |
| Tumor differentiation | Well differentiated | 11 | 14 |
| Moderately differentiated | 58 | 74 | |
| Poorly differentiated | 9 | 11 | |
| Histological type | Adenocarcinoma | 57 | 73 |
| Mucinous adenocarcinoma | 21 | 27 | |
| Nodal status | Negative | 49 | 63 |
| Positive | 29 | 37 | |
| Necrosis | Absent | 71 | 91 |
| Present | 7 | 9 | |
| Lymphatic permeation | Absent | 62 | 80 |
| Present | 16 | 20 | |
| Vascular permeation | Absent | 74 | 95 |
| Present | 4 | 5 | |
| Perineural invasion | Absent | 64 | 82 |
| Present | 14 | 18 | |
| Lymphocytic stromal response | Absent | 63 | 81 |
| Present | 15 | 19 | |
| Pre-operative serum CEA levels (ng/ml) (N=81) | <5 | 35 | 45 |
| ≥ 5 | 43 | 55 | |
| Treatment given | Surgery alone | 14 | 18 |
| S+CT | 45 | 58 | |
| S+RT | 15 | 19 | |
| S+CT+RT | 4 | 5 | |
| Recurrence (N=63) | Absent | 57 | 90 |
| Present | 6 | 10 | |
| Survival (N=78) | Alive | 62 | 80 |
| Mortality | 16 | 20 |
Table 1: Demographic details of CRC patients.
Clinical and pathological parameters
When the patient’s dietary patterns were correlated with various clinical parameters such as age, sex, habit, family history and tumor site, no statistically significant correlation was established. A similar trend was observed for various pathological parameters as shown in Table 2. In patients who presented at an early or advanced stage, the prevalence of vegetarianism was higher than in patients who had a mixed dietary pattern (p=0.063). In comparison to 33% (n=15) of the patients with a mixed dietary pattern, 67% (n=31) of the vegetarian patients developed early CRC. Furthermore, compared to 19% (n=06) of patients on a mixed diet, 81% (n=26) of the vegetarian patients also experienced advanced CRC.
| Characteristics | N | Diet | χ2 | r | P | |
|---|---|---|---|---|---|---|
| Vegetarian N (%) | Mixed N (%) | |||||
| Age (years) | ||||||
| <55 | 34 | 25 (73) | 09 (27) | 0.006 | 0.009 | 0.938 |
| >55 | 44 | 32 (73) | 12 (27) | |||
| Sex | ||||||
| Female | 35 | 28 (80) | 07 (20) | 1.547 | 0.141 | 0.219 |
| Male | 43 | 29 (67) | 14 (33) | |||
| Habit | ||||||
| No | 41 | 32 (78) | 09 (22) | 1.086 | 0.118 | 0.304 |
| Yes | 37 | 25 (68) | 12 (32) | |||
| Family history | ||||||
| No | 76 | 57 (75) | 19 (25) | 2.411 | 0.267 | 0.12 |
| Yes | 2 | 00 (00) | 02 (100) | |||
| Tumor site | ||||||
| Colon | 46 | 33 (72) | 13 (28) | 0.102 | -0.036 | 0.753 |
| Rectum | 32 | 24 (75) | 08 (25) | |||
| TNM stage | ||||||
| I | 9 | 04 (44) | 05 (56) | |||
| II | 37 | 27 (73) | 10 (27) | 4.837 | -0.212 | 0.063 |
| III | 32 | 26 (81) | 06 (19) | |||
| Early (I+II) | 46 | 31 (67) | 15 (33) | 1.842 | -0.154 | 0.179 |
| Advanced (III) | 32 | 26 (81) | 06 (19) | |||
Table 2: Correlation of diet with clinical and pathological parameters in CRC patients.
Genetic mutation and clinicopathological correlation
Genetic mutations in KRAS codons 12 and 13 were analyzed using PCR-RFLP in primary tumor samples from patients with CRC. A total of 23% (n=18) of the patients showed the mutant genotype while 77% (n=60) of the patients had the wild-type genotype of KRAS codon 12. For KRAS codon 13, all patients had wild-type genotype. The correlation between these mutations and clinical and pathological parameters was studied in patients with CRC. A statistically significant correlation was found between KRAS codon 12 mutations and necrosis (p=0.025). In addition, a noticeable trend was observed for tumor differentiation (p=0.06) and pre-operative CEA level (p=0.087) (Table 3).
| Characteristics | N | KRAS codon 12 mutation | χ2 | r | P | |
|---|---|---|---|---|---|---|
| Wild type N (%) | Mutant type N (%) | |||||
| Diet | ||||||
| Vegetarian | 57 | 44 (77) | 13 (23) | 0.009 | 0.011 | 0.927 |
| Mixed | 21 | 16 (76) | 05 (24) | |||
| Tumor site | ||||||
| Colon | 46 | 36 (78) | 10 (22) | 0.113 | 0.038 | 0.741 |
| Rectum | 32 | 24 (75) | 08 (25) | |||
| Tumor differentiation | ||||||
| Well | 11 | 06 (55) | 05 (46) | 4.015 | -0.214 | 0.06 |
| Moderate | 58 | 46 (79) | 12 (21) | |||
| Poor | 9 | 08 (89) | 01 (11) | |||
| Necrosis | ||||||
| Absent | 71 | 57 (80) | 14 (20) | 5.027 | 0.254 | 0.025 |
| Present | 7 | 03 (43) | 04 (57) | |||
| Pre-op circulating CEA (ng/ml) (N=131) | ||||||
| <5.0 | 35 | 30 (86) | 05 (14) | 2.961 | 0.196 | 0.087 |
| ≥ 5 | 42 | 29 (69) | 13 (31) | |||
Table 3: Correlation of KRAS codon 12 and 13 mutation with clinical and pathological parameters in CRC patients.
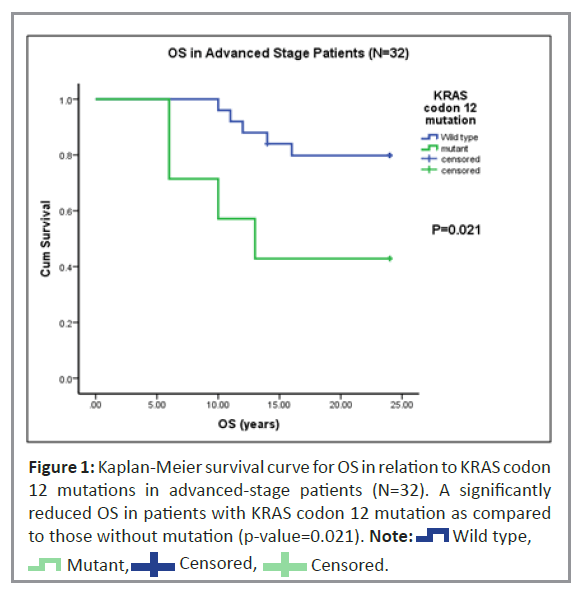
Univariate analysis was performed to study Recurrence-Free Survival (RFS) and Overall Survival (OS) of patients with KRAS codon 12 mutations. In our study, 80% (n=62) of the 78 patients were alive while 20% (n=16) died at the time of follow-up. Of the 63 patients studied, only six had a recurrence of CRC (Table 1). In patients with advanced disease stage, Kaplan-Meier univariate survival analysis for OS revealed a statistically higher (p=0.02) incidence of death (Figure 1). However, no significant trend was observed for RFS in patients with the KRAS codon 12 mutations.
Protein mutation and expression
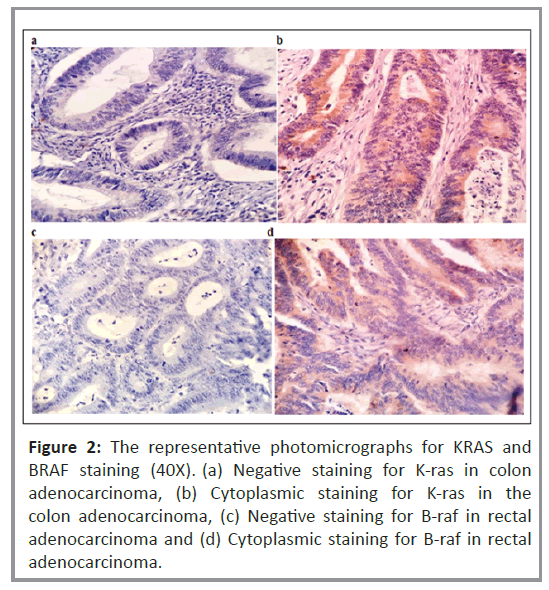
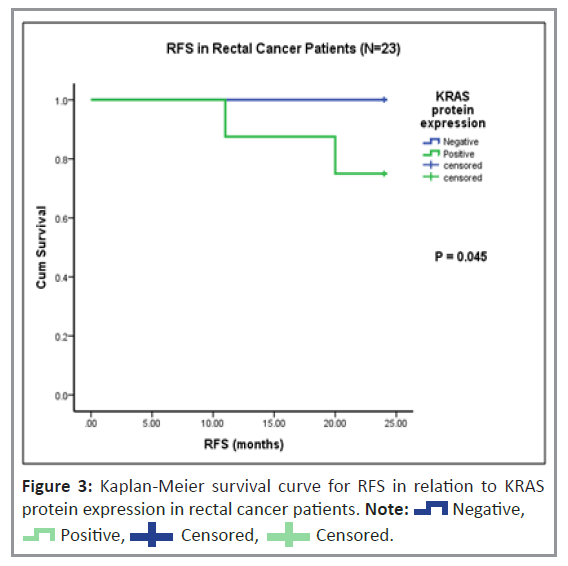
Histopathological analysis was performed to examine the cytoplasmic expression of the K-ras and B-raf proteins. Figure 2 shows the immunoreactivity-based detection of K-ras (Figures 2a and 2b) and B-raf (Figures 2c and 2d) protein expression in 35% (n=27) and 63% (n=49) of patients, respectively. The correlation of K-ras and B-raf protein expression with clinical and pathological parameters in patients with CRC was investigated. As shown in Table 4, there was no correlation between K-ras protein expression and clinical or pathological parameters, including variations between the vegetarian and mixed diets. In a subgroup of rectal cancer patients, Kaplan-Meier univariate survival analysis for RFS and OS revealed a substantially higher incidence of disease relapse in patients with positive K-ras protein expression than in those with negative K-ras protein expression (p=0.045), (Figure 3). However, no significant relationship was observed for OS.
| Characteristics | N | KRAS protein expression | χ2 | r | P | |
|---|---|---|---|---|---|---|
| Negative N (%) | Positive N (%) | |||||
| Diet | ||||||
| Vegetarian | 57 | 38 (67) | 19 (33) | 0.154 | 0.044 | 0.7 |
| Mixed | 21 | 13 (62) | 08 (38) | |||
| Tumor site | ||||||
| Colon | 46 | 28 (61) | 18 (39) | 1.01 | -0.114 | 0.321 |
| Rectum | 32 | 23 (72) | 09 (28) | |||
| Nodal status | ||||||
| Negative | 49 | 30 (61) | 19 (39) | 1.008 | -0.114 | 0.322 |
| Positive | 29 | 21 (72) | 08 (28) | |||
| TNM stage | ||||||
| I | 9 | 05 (56) | 04 (44) | |||
| II | 37 | 23 (62) | 14 (38) | 1.15 | -0.121 | 0.29 |
| III | 32 | 23 (72) | 09 (28) | |||
| Early (I+II) | 46 | 28 (61) | 18 (39) | 1.01 | -0.114 | 0.321 |
| Advanced (III) | 32 | 23 (72) | 09 (28) | |||
| Dukes’ stage | ||||||
| B | 46 | 28 (61) | 18 (39) | 1.01 | -0.114 | 0.321 |
| C | 32 | 23 (72) | 09 (28) | |||
| Tumor differentiation | ||||||
| Well | 11 | 07 (64) | 04 (36) | 0.691 | -0.069 | 0.549 |
| Moderate | 58 | 37 (64) | 21 (36) | |||
| Poor | 9 | 07 (78) | 02 (22) | |||
| Histologic type | ||||||
| Adenocarcinoma | 57 | 36 (63) | 21 (37) | 0.464 | -0.077 | 0.502 |
| Mucinous/Signet ring cell | 21 | 15 (71) | 06 (29) | |||
| Lymphatic permeation | ||||||
| Absent | 62 | 40 (65) | 22 (36) | 0.101 | -0.036 | 0.755 |
| Present | 16 | 11 (69) | 05 (31) | |||
| Vascular permeation | ||||||
| Absent | 74 | 49(66) | 25(34) | 0.441 | 0.075 | 0.513 |
| Present | 4 | 02(50) | 02(50) | |||
| Lymphocytic stromal response | ||||||
| Absent | 63 | 41 (65) | 22 (35) | 0.013 | -0.013 | 0.909 |
| Present | 15 | 10 (67) | 05 (33) | |||
| Perineural invasion | ||||||
| Absent | 64 | 42 (66) | 22 (34) | 0.009 | 0.011 | 0.925 |
| Present | 14 | 09 (64) | 05 (36) | |||
| Necrosis | ||||||
| Absent | 71 | 46 (65) | 25 (35) | 0.124 | -0.04 | 0.729 |
| Present | 7 | 05 (71) | 02 (29) | |||
| Pre-op circulating CEA (ng/ml) (N=77) | ||||||
| < 5.0 | 35 | 24 (69) | 11 (31) | 0.157 | 0.045 | 0.697 |
| ≥ 5.0 | 42 | 27 (64) | 15 (36) | |||
Table 4: Correlation of KRAS protein expression with clinicopathological parameters in CRC patients.
Figure 2: The representative photomicrographs for KRAS and BRAF staining (40X). (a) Negative staining for K-ras in colon adenocarcinoma, (b) Cytoplasmic staining for K-ras in the colon adenocarcinoma, (c) Negative staining for B-raf in rectal adenocarcinoma and (d) Cytoplasmic staining for B-raf in rectal adenocarcinoma.
The correlation of B-raf protein expression with clinical parameters in CRC patients revealed that vegetarians had higher B-raf protein expression than those with a mixed diet. B-raf protein expression was detected in 68% (n=39) of 57 vegetarian patients and 48% (n=10) of 21 patients on a mixed diet. Compared to pathological parameters, B-raf protein expression was significantly associated with positive nodal disease (p=0.020). Furthermore, the immune reactivity of B-raf protein was significantly higher in patients with perineural invasion (86%) than in those without B-raf protein 58%, (p=0.051), as well as in patients with ≥ 5.0 ng/ ml pre-operative CEA levels (76%) as compared to those with <5.0 ng/ml pre-operative CEA levels 46%, (p=0.006). Thus, higher B-raf positivity was more frequently associated with poor prognostic factors, such as perineural invasion, positive lymph nodes and high circulating CEA levels (>5.0 ng/ml) (Table 5).
| Characteristics | N | BRAF protein expression | χ2 | r | P | |
|---|---|---|---|---|---|---|
| Negative N (%) | Positive N (%) | |||||
| Diet | ||||||
| Vegetarian | 57 | 18 (32) | 39 (68) | 2.843 | -0.191 | 0.094 |
| Mixed | 21 | 11 (52) | 10 (48) | |||
| Tumor site | ||||||
| Colon | 46 | 18 (39) | 28 (61) | 0.183 | 0.048 | 0.674 |
| Rectum | 32 | 11 (34) | 21 (66) | |||
| Nodal status | ||||||
| Negative | 49 | 23 (47) | 26 (53) | 5.374 | 0.262 | 0.02 |
| Positive | 29 | 06 (21) | 23 (79) | |||
| TNM stage | ||||||
| I | 9 | 03 (33) | 06 (67) | |||
| II | 37 | 18 (49) | 19 (51) | 4.173 | 0.164 | 0.151 |
| III | 32 | 08 (25) | 24 (75) | |||
| Early (I+II) | 46 | 21 (46) | 25 (54) | 3.446 | 0.21 | 0.065 |
| Advanced (III+IV) | 32 | 08 (25) | 24 (75) | |||
| Dukes’ stage | ||||||
| B | 46 | 21 (46) | 25 (54) | 3.446 | 0.21 | 0.065 |
| C | 32 | 08 (25) | 24 (75) | |||
| Tumor differentiation | ||||||
| Well | 11 | 03 (27) | 08 (72) | 3.993 | -0.195 | 0.087 |
| Moderate | 58 | 20 (34) | 38 (66) | |||
| Poor | 9 | 06 (67) | 03 (33) | |||
| Perineural invasion | ||||||
| Absent | 64 | 27 (42) | 37 (58) | 3.829 | 0.222 | 0.051 |
| Present | 14 | 02 (14) | 12 (86) | |||
| Pre-op circulating CEA (ng/ml) (N=131) | ||||||
| < 5.0 | 35 | 19 (54) | 16 (46) | 7.552 | 0.313 | 0.006 |
| > 5.0 | 42 | 10 (24) | 32 (76) | |||
Table 5: Correlation of BRAF protein expression with clinical and pathological parameters in CRC patients.
Discussion
The prevalence of colorectal cancer is rising worldwide and is strongly linked to changing lifestyles and food habits. According to epidemiological research, modifiable risk factors, such as food, can prevent approximately half of the risk of colon cancer [13]. Researchers have discovered a positive link between the risk of colorectal cancer and processed meat and preserved foods, whereas consumption of vitamins, minerals and general vegetable/fruit/fiber, reduces the risk of colorectal cancer [14]. Such vegetarian eating patterns are often associated with a lower body mass index, which in turn results in reduced fat accumulation and there is substantial evidence linking higher adiposity to an increased risk of colon cancer [15]. However, in our study, no significant correlation between diet and CRC was found with any clinicopathological or demographic parameter. Further, among vegetarian patients, the majority presented in the early and advanced stages of CRC in contrast to patients with mixed dietary patterns.
Colorectal cancer develops across several stages because of the accumulation of various genetic mutations. Ras and Raf, which are crucial intermediates in the RAS-mediated signaling cascade, are the most significant. Ras proteins are proto-oncogenes that are frequently mutated in human cancer. They are encoded by three ubiquitously expressed genes: HRAS, KRAS and NRAS [16].
The present study examined KRAS codon 12 and 13 mutations using PCR-RFLP and indicated that 23% (n=18) of patients had KRAS codon 12 mutations, while all patients had only wild-type KRAS codon 13. Similar studies have reported the prevalence of KRAS mutations in 42.8% of patients with metastatic CRC in an Indian population [17]. Of all KRAS mutations, a mutation in codon 12 was the most common, followed by mutations in codons 13 and 61 [18]. These variations in KRAS mutation patterns may be due to racial differences and etiological factors. Given the results of several clinical trials, screening for KRAS mutations in codons 12 and 13 for metastatic colorectal cancer treatment has been recommended.
With regard to clinicopathological parameters, the present study showed a significant correlation between KRAS codon 12 mutation and necrosis. Furthermore, a trend of a higher incidence of KRAS codon 12 mutations was found in patients with welldifferentiated tumors than in those with moderately and poorly differentiated tumors. Studies have shown that well-differentiated metastatic CRC tumors have significantly more mutation positivity than moderately and poorly differentiated tumors. In contrast, KRAS mutations are significantly associated with moderately and poorly differentiated colorectal adenocarcinoma [19]. Another study found that peritoneal metastasis, liver-peritoneum metastases and multi-organ metastases were notably related to KRAS codon 12 mutations, but not codon 13 mutations, as compared to all wild types. These findings may help in predicting prognosis and selecting better treatment strategies [20].
Hence, the present study focused on the prognostic and predictive value of KRAS codon 12 and 13 mutation status in CRC patients. Additionally, we examined the role of KRAS codon 12 and 13 mutations in early and advanced-stage patients as well as in the subgroups of colon and rectal cancer patients. The study showed a significant reduction in OS with KRAS codon 12 mutations compared to KRAS wild-type in the advanced stage and prominently in advanced-stage rectal cancer patients. Also, a trend of reduced OS was noted with KRAS codon12 mutant type in advanced-stage patients treated with adjuvant therapy. Accordingly, patients with KRAS mutations had significantly decreased median survival times compared to patients with wildtype KRAS genes in CRC.
Cytoplasmic expression of K-ras and B-raf proteins was examined using immunohistology. None of the clinicopathological parameters was found to be significantly correlated with K-ras protein expression. However, B-raf protein expression was significantly higher in vegetarians as compared to those with a mixed diet. B-raf immunopositivity was significantly higher in patients with perineural invasion, high CEA levels and positive nodal disease.
The expression levels of K-ras and its prognostic evaluation in patients with CRC remain unknown. To the best of our knowledge, this is the first study to demonstrate the prognostic and predictive roles of K-ras immunohistopathological localization in CRC. We observed that the subgroup of rectal cancer patients showed significantly reduced RFS with K-ras positive expression compared to K-ras negative expression (p=0.045). A similar trend of reduced RFS was observed with positive K-ras expression in the subgroup of patients with rectal cancer treated with adjuvant therapy (p=0.073).
Hence, the present study demonstrated that positive cytoplasmic K-ras protein expression could be a useful biomarker for predicting unfavorable prognosis and treatment response in patients with rectal cancer.
Limitations
Further studies with larger patient groups including nonvegetarian populations and longer follow-up periods are needed to better understand the prognostic and predictive role of KRAS and BRAF mutations and K-ras and B-raf protein expression in CRC cases. This would aid in the determination of CRC prevention, therapeutic response and prognosis of CRC patients.
Conclusion
Colorectal cancer is the most prevalent gastrointestinal malignancy worldwide, affecting both males and females. Dietary pattern has been postulated to affect the demographics of CRC patients. Unfortunately, most CRC tumors are benign, grow slowly, and do not manifest symptoms until they grow larger. Most genetic alterations observed in colorectal tumorigenesis are associated with K-ras and B-raf. These mutations most commonly occur in KRAS codons 12 and 13 and render the therapeutic modulation of epidermal growth factor receptor irrelevant.
The present study did not show any significant clinicopathological variations among vegetarian and mixed-diet patients. However, a trend of an advanced stage among vegetarian patients was observed in this study. The study also demonstrated a correlation between patient prognostic factors and B-raf expression as well as a slight positive association of B-raf expression in vegetarian patients. KRAS codon 12 and 13 mutations as well as K-ras and B-raf protein expression could be useful prognostic and predictive markers in CRC patients. These data need to be validated in a larger cohort to potentially influence treatment decisions.
Author Contributions
Dr. Nikhil Garg was responsible for the conception and design, analysis and interpretation of the data. Dr. Chamanjot Singh assisted in the drafting of the manuscript or revising it critically for intellectual content. The author has approved the final version of the manuscript to be published.
Data Availability
The data that support the findings of this study are available on request from the corresponding author Dr. Nikhil Garg upon reasonable request.
Declarations
Ethical approval
The ethical approval was taken from the institutional ethics committee of ‘The Gujarat Cancer & Research Institute (GCRI)’ which is registered under the Central Licensing Authority (Central Drugs Standard Control Organization) with registration No ECR/41/Inst/GJ/2013/RR-19.GCRI/GCS.
Conflict of Interest
The author declares no conflict of interest.
Competing Interest
The authors declare there are no financial conflicts of interest to disclose.
References
- Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB (2019) Colorectal cancer. Lancet 394: 1467-1480.
[Crossref], [Google Scholar], [Indexed]
- Schliemann D, Ramanathan K, Matovu N, Neill CO, Kee F, et al. (2021) The implementation of colorectal cancer screening interventions in low-and middle-income countries: A scoping review. BMC Cancer 21: 1125.
[Crossref], [Google Scholar], [Indexed]
- Schreuders EH, Ruco A, Rabeneck L, Schoen RE, Sung JJY, et al. (2015) Colorectal cancer screening: A global overview of existing programmes. Gut 64: 1637-1649.
[Crossref], [Google Scholar], [Indexed]
- Hamada T, Nowak JA, Masugi Y, Drew DA, Song M, et al. (2019) Smoking and risk of colorectal cancer sub-classified by tumor-infiltrating T cells. J Natl Cancer Inst 111: 42-51.
[Crossref], [Google Scholar], [Indexed]
- Xi Y, Xu P (2021) Global colorectal cancer burden in 2020 and projections to 2040. Transl Oncol 14: 101174.
[Crossref], [Google Scholar], [Indexed]
- Song M, Garrett WS, Chan AT (2015) Nutrients, foods and colorectal cancer prevention. Gastroenterology 148: 1244-1260.
[Crossref], [Google Scholar], [Indexed]
- Ye P, Xi Y, Huang Z, Xu P (2020) Linking obesity with colorectal cancer: Epidemiology and mechanistic insights. Cancers 12: 1408.
[Crossref], [Google Scholar], [Indexed]
- Oikonomou E, Koustas E, Goulielmaki M, Pintzas A (2014) BRAF vs RAS oncogenes: Are mutations of the same pathway equal? Differential signalling and therapeutic implications. Oncotarget 5: 11752-11777.
[Crossref], [Google Scholar], [Indexed]
- Zhu G, Pei L, Xia H, Tang Q, Bi F (2021) Role of oncogenic KRAS in the prognosis, diagnosis and treatment of colorectal cancer. Mol Cancer 20: 143.
[Crossref], [Google Scholar], [Indexed]
- Barras D (2015) BRAF mutation in colorectal cancer: An update. Biomark Cancer 7: 9-12.
[Crossref], [Google Scholar], [Indexed]
- van Brummelen EMJ, de Boer A, Beijnen JH, Schellens JHM (2017) BRAF mutations as predictive biomarker for response to anti-EGFR monoclonal antibodies. Oncologist 22: 864-872.
[Crossref], [Google Scholar], [Indexed]
- Midthun L, Shaheen S, Deisch J, Senthil M, Tsai J, et al. (2019) Concomitant KRAS and BRAF mutations in colorectal cancer. J Gastrointest Oncol 10: 577-581.
[Crossref], [Google Scholar], [Indexed]
- Zhou E, Rifkin S (2021) Colorectal cancer and diet: Risk versus prevention, is diet an intervention?. Gastroenterol Clin North Am 50: 101–111.
[Crossref], [Google Scholar], [Indexed]
- Azeem S, Gillani SW, Siddiqui A, Jandrajupalli SB, Poh V, et al. (2015) Diet and colorectal cancer risk in Asia--a systematic review. Asian Pac J Cancer Prev 16: 5389-5396.
[Crossref], [Google Scholar], [Indexed]
- Orlich MJ, Singh PN, Sabaté J, Fan J, Sveen L, et al. (2015) Vegetarian dietary patterns and the risk of colorectal cancers. JAMA Intern Med 175: 767-776.
[Crossref], [Google Scholar], [Indexed]
- Arrington AK, Heinrich EL, Lee W, Duldulao M, Patel S, et al. (2012) Prognostic and predictive roles of KRAS mutation in colorectal cancer. Int J Mol Sci 13: 12153-12168.
[Crossref], [Google Scholar], [Indexed]
- Veldore VH, Rao MR, Prabhudesai SA, Tejaswi R, Kakara S, et al. (2014) Prevalence of KRAS mutations in metastatic colorectal cancer: A retrospective observational study from India. Indian J Cancer 51: 531-537.
[Crossref], [Google Scholar], [Indexed]
- Mehra S, Tiwari AK, Mehta SP, Sachdev R, Rajvanshi C, et al. (2022) The utility of the ion torrent PGM next generation sequencing for analysis of the most commonly mutated genes among patients with colorectal cancer in India. Indian J Cancer 59: 218-222.
[Crossref], [Google Scholar], [Indexed]
- Bisht S, Ahmad F, Sawaimoon S, Bhatia S, Das BR (2014) Molecular spectrum of KRAS, BRAF, and PIK3CA gene mutation: Determination of frequency, distribution pattern in Indian colorectal carcinoma. Med Oncol 31: 124.
[Crossref], [Google Scholar], [Indexed]
- He K, Wang Y, Zhong Y, Pan X, Si L, et al. (2020) KRAS Codon 12 mutation is associated with more aggressive invasiveness in synchronous metastatic Colorectal Cancer (mCRC): Retrospective research. Onco Targets Ther 13: 12601-12613.
[Crossref], [Google Scholar], [Indexed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences