Tumor Location may Affect Total Mesorectal Excision Quality
Marissa A Mendez, Rebecca Wilcox, Peter W Callas and Peter Cataldo
Marissa A Mendez1*, Rebecca Wilcox2, Peter W Callas3 and Peter Cataldo4
1University of Missouri Kansas-City, Truman Medical Center, 2301 Holmes Street, Kansas City, MO 64108, USA
2Department of Pathology, University of Vermont College of Medicine, 89 Beaumont Avenue, Courtyard at Given S269, Burlington, VT 05405, USA
3Medical Biostatistics Department, University of Vermont College of Medicine, 109 Votey Hall, 33 Colchester Avenue, Burlington, VT 05405-0156, USA
4Department of Surgery, University of Vermont College of Medicine, Fletcher House 301, 111 Colchester Avenue, Burlington, VT 05401, USA
- *Corresponding Author:
- Marissa A Mendez
MD, Surgery Resident, University of Missouri Kansas-City, Truman Medical Center, 2301 Holmes Street, Kansas City, MO 64108, USA
Tel: 816-404-5372
E-mail: mendezmar@umkc.edu
Received date: December 21, 2015; Accepted date: January 21, 2016; Published date: January 27, 2016
Citation: Mendez MA, Wilcox R, Callas PW, et al. Tumor Location may Affect Total Mesorectal Excision Quality. Colorec Cancer.2016, 2:1. doi: 10.21767/2471-9943.100012
Abstract
Purpose: Total mesorectal excision (TME) is standard of care for patients with resectable rectal cancer. Post-operatively, specimens are graded as complete, nearly complete, or incomplete. It is known that incomplete mesorectal excisions are associated with increased local recurrence. This study looks at factors which may predict incomplete resections.
Methods: This retrospective medical record review looked at patients who underwent planned total mesorectal excision for rectal cancer from January 2012 to January 2015. Data was collected for the following variables: tumor height, tumor location, history of radiation therapy, history of prior pelvic surgery, history of neoadjuvant therapy, laparoscopic or open surgery. Patients were compared based on total mesorectal excision quality. Statistical analysis was done with Fisher’s exact tests and ANOVAs.
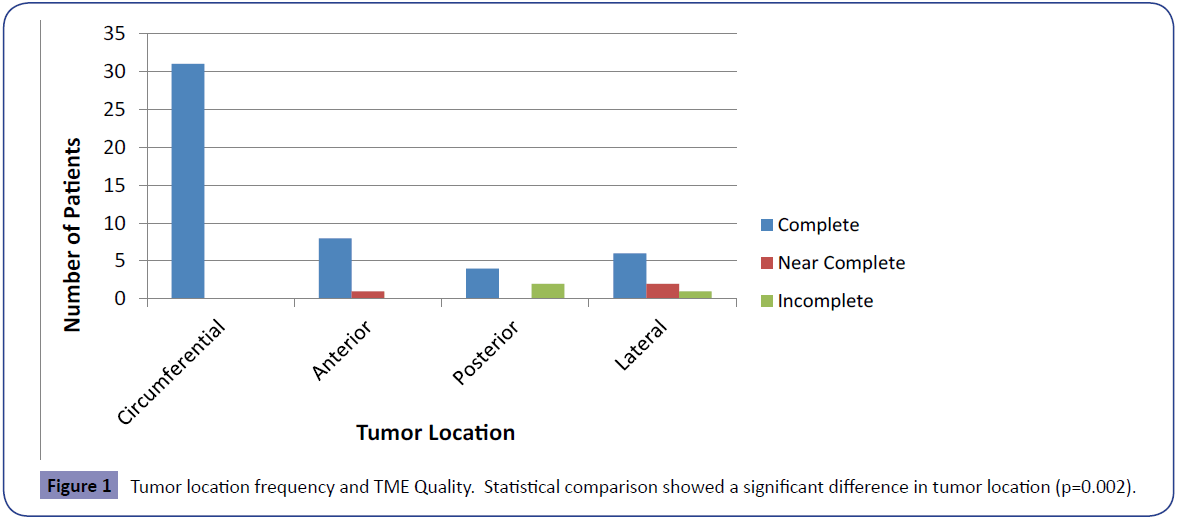
Results: 57 patient charts were reviewed and one patient was excluded. Out of this 56 patient cohort, 89% of specimens were complete, 5% were near complete and 5% were incomplete. Tumor location was the only variable which was significantly different among the three groups (p=0.002). Incomplete specimens came from tumors that were posterior (67%) and lateral (33%). Near complete specimens came from tumors that were anterior (33%) and lateral (67%). Out of 31 patients with circumferential tumors, 100% had complete TME’s.
Conclusions: Tumor location may affect TME quality. In this present study, circumferential tumors were associated with a complete TME whereas other tumor locations presented a risk for nearly complete or incomplete TME. Care should be taken during the dissection and the experienced surgeon should be involved in the surgery of tumors that are not circumferential.
Keywords
Total mesorectal excision; Pathology; Grading; Recurrence
Introduction
The treatment for rectal cancer has evolved significantly over the last three decades. Despite advances in technology and treatment algorithms, precise surgical resection remains essential in minimizing tumor recurrence. That technique includes resection of both the tumor and the mesorectum. Currently, treatment involves a multidisciplinary approach consisting of a range of experts including: surgeons, radiologists, pathologists, medical oncologists, and radiation oncologists. About 84% of patients with rectal cancer undergo surgical intervention whether it be for palliative or curative intent depending on the stage of the tumor [1]. Total mesorectal excision (TME), originally proposed by Heald et al. is currently the standard of care for patients with resectable rectal cancer [2]. For patients with tumors in the lower twothirds of the rectum, TME is the gold standard. For tumors in the upper third of the rectum, tumor specific mesorectal excision is also effective. Through the years it has become clear that variability in surgical techniques resulting in incomplete tumor excision can affect outcomes in terms of local recurrence and patient survival [3,4].
The mesorectum is the layer of fatty soft tissue surrounding the rectum which contains lymphovasular and neural tissue [2]. In the mesorectum, tumor may spread via lymphatics, perineural invasion or via tumor deposits from the primary tumor. In order to perform TME, the surgeon must identify and dissect the plane posterior to the fascia propria of the rectum and anterior to the presacral fascia. This will result in accurate resection of the mesorectum with minimal bleeding or trauma to the nerves responsible for bladder and sexual function. Care must be taken to not stray into or out of the mesorectal envelope [5].
Since the introduction of TME by Heald et al. several studies, including large multi-center randomized control trials, have gone on to further describe effective treatment strategies for rectal carcinoma [6-9]. In terms of surgical technique, 5-year local recurrence rates have been shown to drop from 15-45% with non-standardized rectal cancer resections to less than 8% with total mesorectal excision [6]. Furthermore, the Dutch TME trial and Swedish Rectal Cancer Trial showed that the addition of preoperative radiation in conjunction with TME resulted in even further decreased local recurrence and possibly increased overall survival [6,8,9].
Post operatively, specimens are evaluated both microscopically and macroscopically by a pathologist in order to determine the quality of the resection [10,11]. Specimens are given a grade of Incomplete, Nearly Complete, or Complete based on several factors including: regularity of the circumferential resection margin, presence of coning, defects in the mesorectum, and integrity of posterior and anterior visceral fascia [12]. Many institutions have adopted synoptic pathology reporting for colorectal cancer, which is essentially a checklist for specimen analysis that has been shown to improve completeness and accuracy of assessment of tissue specimens [13,14]. This type of reporting was introduced to our institution beginning in 2012.
In order to give patients the best chance at disease free survival, performing accurate total mesorectal excision and achieving a mesorectum grade of “complete,” is critical. Therefore, by studying which factors may contribute to an “incomplete” specimen, we may gain insight into how to better our surgical approach or technique.
Methods
This retrospective study, involving a medical chart review, was approved by the Institutional Review Board at the University of Vermont. The aim was to perform a medical record review of patients who have previously undergone planned total mesorectal excision for rectal cancer in order to determine if any of the following factors may have contributed to the pathologic quality of the TME: tumor height, tumor location, history of radiation therapy, history of previous pelvic or abdominal surgery, laparoscopic versus open technique, and low anterior resection versus abdominoperineal resection. Other factors that were evaluated in terms of their effect on TME quality were TNM stage, circumferential resection margin (CRM), and neoadjuvant chemotherapy.
Study population
All patients who underwent rectal cancer excisions beginning in January 2012 (when our institution implemented synoptic pathology reporting for colorectal carcinoma) through January 2015 were included. Patient exclusion criteria included: primary tumor of the colon, surgery performed for recurrent rectal cancer, and patients undergoing Transanal Endoscopic Microsurgery. Patients must have been 18 years or older at the time of surgery in order to be included in the study.
Data extraction and variables
Patient data was retrieved from synoptic pathology reports, operative reports, and perioperative office notes from the electronic medical record at the University of Vermont Medical Center. Patient information was de-identified prior to entry into our database.
The following baseline and clinical data were collected: age, sex, race, history of previous abdominal or pelvic operations, history of pelvic radiation, tumor height, tumor location, laparoscopic versus open surgery, Low Anterior Resection versus Abdominoperineal Resection, TNM stage, history of neoadjuvant chemo/radiation therapy, margin status including CRM, and the pathologic quality of the mesorectum. Tumor height was defined by distance in centimeters from the dentate line.
All total mesorectal excisions were performed by 1 of 4 fellowship trained colorectal surgeons at our institution, of whom the average time out of fellowship was 18 years. All specimens were graded independently by pathologist assistants with second opinion by a fellowship trained GI pathologist on any case that was not “complete.” Status of circumferential resection margin was documented and completeness of mesorectum was scored as described by grade of complete, nearly complete, or incomplete (Table 1).
| Mesorectum | Defects | Coning | CRM | |
|---|---|---|---|---|
| Complete | Intact, smooth | Not deeper than 5mm | None | Smooth, regular |
| Nearly Complete | Moderate bulk, irregular | No visible muscularis propria | Moderate | Irregular |
| Incomplete | Little Bulk | Down to muscularis propria | Moderate-marked | Irregular |
Table 1: Grading of quality of mesorectum following Total Mesorectal Excision for rectal cancer [7].
Statistical analysis
Overall, variables were statistically analyzed and then patients were distributed into groups according to specimen quality: incomplete, nearly complete, and complete. Statistical analysis was performed to compare the three groups and then another analysis was done which grouped the “completes” and “nearly completes” together and compared this combined group to the “incompletes.” Because of a small sample size, comparisons between these groups were done with Fisher’s exact tests. ANOVA was used to determine if there was a difference in age, tumor heights, or number of lymph nodes removed between the three groups. Odds ratios were not performed due to the small sample size and numbers of incomplete specimens.
Results
A total of 57 patient charts were reviewed. One patient was excluded due to undergoing Transanal Endoscopic Microsurgery (TEM). Mean age overall was 59 (SD=13) and there were 37 (66%) males and 19 (34%) females. There were 36 (64%) patients who had a prior history of radiation and 22 (39%) had a prior history of abdominal or pelvic surgery (Table 2). There were 35 (62%) patients who underwent neoadjuvant therapy for treatment of their rectal cancer.
| TME Quality | ||||||
|---|---|---|---|---|---|---|
| Patient Characteristics | N | Complete N (%) | Near complete N (%) | Incomplete N (%) | p- value | |
| Sex | Male | 37 | 33 (89%) | 1 (3%) | 3 (8%) | 0.26 |
| Female | 19 | 17 (89%) | 2 (11%) | 0 (0%) | ||
| Race | White | 51 | 45 (88%) | 3 (6%) | 3 (6%) | 1.00 |
| Other | 5 | 5 (100%) | 0 (0%) | 0 (0%) | ||
| History of radiation | No | 20 | 18 (90%) | 1 (5%) | 1 (5%) | 1.00 |
| Yes | 36 | 32 (89%) | 2 (6%) | 2 (6%) | ||
| History of abdominal or pelvic surgery | No | 34 | 30 (88%) | 2 (6%) | 2 (6%) | 1.00 |
| Yes | 22 | 20 (91%) | 1 (5%) | 1 (5%) | ||
| Total | 56 | 50 (89%) | 3 (5%) | 3 (5%) |
Table 2: Patient characteristics and TME quality.
Out of the total 56 rectal resections, 48 (86%) procedures were done open, 6 (11%) were done laparoscopic, and 2 (3%) were laparoscopic converted to open. There were 43 (77 %) low anterior resections and 13 (23%) abdominoperineal resections (Table 3).
| TME Quality | |||||
|---|---|---|---|---|---|
| Surgical Technique | N | Complete N (%) | Near complete N (%) | Incomplete N (%) | p- value |
| Laparoscopic | 6 | 5 (83%) | 1 (17%) | 0 (0%) | 0.62 |
| Open | 48 | 43 (90%) | 2 (4%) | 3 (6%) | |
| Converted | 2 | 2 (100%) | 0 (0%) | 0 (0%) | |
| Low Anterior Resection | 43 | 39 (91%) | 3 (7%) | 1 (2%) | 0.25 |
| Abdominoperineal Resection | 13 | 11 (85%) | 0 (0%) | 2 (15%) | |
| Lithotomy | 6 | 5 (83%) | 0 (0%) | 1 (17%) | 1.00 |
| Prone | 7 | 6 (86%) | 0 (0%) | 1 (14%) | |
| Total | 56 | 50 (89%) | 3 (5%) | 3 (5%) |
Table 3: Comparisons of surgical technique to TME quality.
Overall the TME specimen quality was 50 (89%) complete, 3 (5%) near complete, and 3 (5%) incomplete. The overall mean tumor height was 7.0 cm (SD=4.5) from the dentate line. There was no significant difference in the mean tumor height between the three groups (p=0.09), which were as follows: 7.0 cm (SD=4.5) for completes, 10.7 cm (SD=3.1) for near completes, and 2.7 cm (SD=1.5) for incompletes. The overall mean number of lymph nodes removed was 15.6 (SD=9.4) nodes. There was also no significant difference in number of nodes removed between the three groups (p=0.65), which were as follows: 16.0 (SD=9.5) from completes, 13.3 (SD=3.8) from near completes, and 11.3 (SD=12.1) from incompletes.
The overall tumor locations were as follows: 9 (16%) anterior, 6 (11%) posterior, 31 (55%) circumferential, 9 (16%) lateral, and 1 (2%) unknown (Table 4). The patient with the unknown tumor location was omitted from further analysis in regards to tumor location. Tumor location was significantly different between the three groups (p=0.002). Incomplete specimens came from tumors that were located posterior (67%) and lateral (33%). Complete specimens came from tumors located circumferentially (63%), anterior (16%), posterior (8%), lateral (12%). Nearly complete specimens came from tumors located lateral (67%) and anterior (33%) (Figure 1).
| TME Quality | |||||
|---|---|---|---|---|---|
| Tumor Location | N | Complete N (%) | Near complete N (%) | Incomplete N (%) | p- value |
| Anterior | 9 | 8 (89%) | 1 (11%) | 0 (0%) | 0.002 |
| Posterior | 6 | 4 (67%) | 0 (0%) | 2 (33%) | |
| Circumferential | 31 | 31 (100%) | 0 (0%) | 0 (0%) | |
| Lateral | 9 | 6 (67%) | 2 (22%) | 1 (11%) | |
| Total | 55 | 49 (89%) | 3 (5%) | 3 (5%) |
Table 4: Tumor location and TME quality.
Circumferential resection margin was positive in 2 (4%) specimens, both of which had complete TME specimen grades. The mean closest margin to tumor was significantly different between the three groups (p=0.03): 1.15 cm for completes, 0.57 cm for near completes and 0.25 cm for incompletes (Table 5). When it came to TNM staging, we looked at differences in TME quality in relation to the level of staging. There were significant differences in TME quality between patients who had no regional lymph node metastasis (N0) and those who had tumor deposits in nonperitonealized pericolic or perirectal tissues (N1c) (p=<0.001). Out of our cohort of patients, the highest tumor stage was T4b and that patient had a complete TME.
| TME Quality | |||||
|---|---|---|---|---|---|
| Complete | Near Complete | Incomplete | p-value | ||
| Closest positive margin (cm) | Mean (SD) | 1.15 (1.10) | 0.57 (0.38) | 0.25 (0.19) | 0.03 |
| Circumferential resection margin | |||||
| Positive N (%) | 2 | 2 (100%) | 0 | 0 | 1.00 |
| Negative N (%) | 54 | 48 (89%) | 3 (6%) | 3 (6%) | |
Table 5: Margins and TME Quality.
Other variables, which had no effect on TME quality, included: tumor height (p=0.09), laparoscopic versus open technique (p=0.62), low anterior resection versus abdominoperineal resection (p=0.25), history of previous radiation (p=1.00), history of abdominal or pelvic surgery (p=1.00), number of lymph nodes removed (0.65), history of neoadjuvant therapy (p=0.80), or sex (p=0.26).
Since patients with complete and nearly complete TME have similar local and overall recurrence rates, these two groups were combined and compared to the incomplete group [15]. Again, tumor location was the only variable which differed significantly among the two groups (p=0.01) (Figure 2). All other variables had no effect on TME quality: tumor height (p=0.09), laparoscopic versus open technique (p=1.00), low anterior resection versus abdominoperineal resection (p=0.13), history of previous radiation (p=1.00), history of abdominal or pelvic surgery (p=1.00), number of lymph nodes (0.42), or sex (p=0.54).
Discussion
Total Mesorectal Excision is the standard of care for patients undergoing curative resection for rectal cancer. The importance of precise surgical technique is paramount [3,4,6-9]. Studies which have looked at the correlation between the pathologic grading of the mesorectum and recurrence rates have found increased local and distant recurrence rates with incomplete TME [15,16]. Maslekar et al. looked at 130 patients and found local recurrence rates to be 41% in patients with incomplete specimens, 6% in patients with nearly complete specimens, and <2% in patients with complete specimens [15]. Nagtegaal et al. looked at 180 patients and found recurrence rates in patients with incomplete versus complete to be 36.1% versus 20.3% respectively [16]. The few studies which have looked at factors that may contribute to incomplete total mesorectal excisions have found variables such as abnormal BMI and narrow pelvic diameter to be predictive [17-19]. There have also been conflicting studies regarding the effects of laparoscopic versus open surgery [3].
This retrospective chart review which looked at 56 patients who underwent total mesorectal excisions for rectal cancer found that 89% of specimens were complete, 5% were near complete and 5% were incomplete. Out of 11 variables analyzed, tumor location was the only one which predicted the quality of TME (p=0.002). Because of similar local recurrence rates between “completes” and “near completes” in previous studies, these two groups were combined and compared to the incomplete group [15]. Results remained similar with tumor location being the only variable to differ significantly between the two groups (p=0.01). Not surprisingly, the closest positive margin was significantly smaller in specimens that were incomplete with an average margin of 0.25 cm in incomplete specimens versus 1.15 cm in complete specimens (p=0.03). Furthermore, no statistically significant difference in the overall quality of the TME was found when we compared cases done laparoscopic, open, and laparoscopic converted to open (p=0.62). This compliments many studies which have found similar local control and long term cancer free survival between the two techniques [20-22].
It has previously been suggested that the amount of soft tissue which surrounds the rectum varies circumferentially and therefore, the location of the tumor may have important prognostic implications [7]. In this present study, all circumferential tumors had complete total mesorectal excisions whereas near complete and incomplete specimens were associated with tumors in other locations such as posterior and lateral. The authors of this study postulate that this could be due to posterior and lateral tumors obscuring the mesorectal envelope or contributing to an already thin mesorectum in these locations and pushing the envelope more posteriorly [3,23]. It is well known that TME is a difficult procedure due to the complicated anatomy of the pelvis with its narrow spaces and surgical planes, but perhaps tumors that are located in these other locations add to the difficulty of dissecting difficult planes while trying to avoid injury of pelvic splanchnic nerves [23].
In conclusion, tumor location may affect the quality of the mesorectal specimens for patients who have undergone total mesorectal excision for rectal cancer. Circumferential tumor predicts a complete TME, whereas other tumor locations seem to represent a risk for incomplete or nearly complete TME. Although this study was limited by small sample size, these findings should be considered in surgical planning and during surgical dissection in order to achieve the best possible outcomes. Surgeons should pay particular attention to careful identification of the mesorectal envelope (and fascia of the rectum) while performing TME when tumors are not circumferential. The authors recommend larger studies to further characterize factors which may contribute to TME quality.
References
- Jessup JM, McGinnis LS, Steele GD, Menck HR, Winchester DP (1996) the National Cancer Data Base. Report on colon cancer. Cancer 78:918-926.
- Heald RJ, Husband EM, Ryall RD (1982) themesorectum in rectal cancer surgery-the clue to pelvic recurrence? The British journal of surgery 69: 613-616.
- Campa-Thompson M, Weir R, Calcetera N, Quirke P, Carmack S (2015) Pathologic processing of the total mesorectal excision. Clinics in colon and rectal surgery 28:43-52.
- Kusters M, Marijnen CA, van de Velde CJ, Rutten HJ, Lahaye MJ, et al. (2010) Patterns of local recurrence in rectal cancer; a study of the Dutch TME trial. European journal of surgical oncology: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology 36: 470-476.
- Taflampas P, Christodoulakis M, de Bree E, Melissas J, Tsiftsis DD (2010) Preoperative decision making for rectal cancer. American journal of surgery 200:426-432.
- Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, et al. (2001) Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. The New England journal of medicine 34:638-646.
- Parfitt JR, Driman DK (2007) the total mesorectal excision specimen for rectal cancer: a review of its pathological assessment. Journal of clinical pathology 60:849-855.
- vanGijn W, Marijnen CA, Nagtegaal ID, Kranenbarg EM, Putter H, et al. (2011) Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. The lancet oncology 12:575-582.
- Folkesson J, Birgisson H, Pahlman L, Cedermark B, Glimelius B, et al. (2005) Swedish Rectal Cancer Trial: long lasting benefits from radiotherapy on survival and local recurrence rate. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 23:5644-5650.
- Quirke P (2003) Training and quality assurance for rectal cancer: 20 years of data is enough. The lancet oncology 4 :695-702
- Quirke P, Durdey P, Dixon MF, Williams NS (1986) Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumour spread and surgical excision. Lancet 2: 996-999
- Simunovic MR, DeNardi FG, Coates AJ, Szalay DA, Eva KW (2014) Product analysis and initial reliability testing of the total mesorectal excision-quality assessment instrument. Annals of surgical oncology 21:2274-2279.
- Beattie GC, McAdam TK, Elliott S, Sloan JM, Irwin ST (2003) Improvement in quality of colorectal cancer pathology reporting with a standardized proforma--a comparative study. Colorectal disease: the official journal of the Association of Coloproctology of Great Britain and Ireland 5:558-562.
- Quirke P, Morris E (2007) Reporting colorectal cancer. Histopathology 50:103-112.
- Maslekar S, Sharma A, Macdonald A, Gunn J, Monson JR, et al. (2007) Mesorectal grades predict recurrences after curative resection for rectal cancer. Diseases of the colon and rectum 50:168-175.
- Nagtegaal ID, van de Velde CJ, van der Worp E, Kapiteijn E, Quirke P, et al. (2002) macroscopic evaluation of rectal cancer resection specimen: clinical significance of the pathologist in quality control. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 20:1729-1734.
- Leonard D, Penninckx F, Fieuws S, Jouret-Mourin A, Sempoux C, et al. (2010) Factors predicting the quality of total mesorectal excision for rectal cancer. Annals of surgery 252:982-988.
- Jeyarajah S, Sutton CD, Miller AS, Hemingway D (2007) Factors that influence the adequacy of total mesorectal excision for rectal cancer. Colorectal disease: the official journal of the Association of Coloproctology of Great Britain and Ireland 9:808-815.
- Deng H, Chen H, Zhao L (2015) Quality of laparoscopic total mesorectal excision: results from a single institution in China. Hepatogastroenterology62:264-7.
- Laurent C, Leblanc F, Wutrich P, Scheffler M, Rullier E (2009) Laparoscopic versus open surgery for rectal cancer: long-term oncologic results. Annals of surgery 250:54-61.
- Leroy J, Jamali F, Forbes L, Smith M, Rubino F, et al. (2004) Laparoscopic total mesorectal excision (TME) for rectal cancer surgery: long-term outcomes. Surgical endoscopy 18:281-289.
- Vennix S, Pelzers L, Bouvy N, Beets GL, Pierie JP, et al. (2014) Laparoscopic versus open total mesorectal excision for rectal cancer. The Cochrane database of systematic reviews 4:Cd005200.
- Havenga K, Grossmann I, DeRuiter M, Wiggers T (2007) Definition of total mesorectal excision, including the perineal phase: technical considerations. Digestive diseases 25:44-50.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences