Bridging Stenting for Stenotic Left-sided Colorectal Tumour Versus Emergency Colectomy
James Tak-Kwan Fung1, Max Wing Yin Chung1, Chun Sing Wong1, Michael Chi Ming Poon1 and Siu Yan Kok2
1.Department of Surgery, Tuen Mun Hospital, Tuen Mun, New Territories, Hong Kong 2Department of Surgery, United Christian Hospital, Kwun Tong, Kowloon, Hong Kong
Published Date: 2022-11-21DOI10.36648/2471-9943.8.5.03
James Tak-Kwan Fung1*and SiuYan Kok2, Max Wing Yin Chung1, Chun Sing Wong1 and Michael Chi Ming Poon1
1Department of Surgery, Tuen Mun Hospital, Tuen Mun, New Territories, Hong Kong
2Department of Surgery, United Christian Hospital, Kwun Tong, Kowloon, Hong Kong
- *Corresponding Author:
- James Tak-Kwan Fung
Department of Surgery,
Tuen Mun Hospital, Tuen Mun, New Territories,
Hong Kong,
E-mail: jamestkfung@protonmail.com
Received date: October 21, 2022, Manuscript No. IPJCC-22-14829; Editor assigned date: October 24, 2022, PreQC No. IPJCC-22-14829 (PQ); Reviewed date: November 04, 2022, QC No IPJCC-22-14829; Revised date: November 14, 2022, Manuscript No. IPJCC-22-14829 (R); Published date: November 21, 2022, DOI: 10.36648/2471-9943.8.5.03
Citation: Fung JTK, Kok SY, Chung MWY, Wong CS, Poon MCM (2022) Bridging Stenting for Stenotic Left-sided Colorectal Tumor Versus Emergency Colectomy. Colore.c Cancer Vol.8 No.5:03
Abstract
Aim
To investigate the oncological outcome of bridging stent insertion compared with emergency colectomy for patient with Left Sided Intestinal Obstruction (LSIO) treated with a curative intent.
Method
A retrospective review of patient record between 1/1/13-31/12/17 was done. Patients admitted with emergency. LSIO with no distant metastasis, who were subsequently treated with intent to cure were recruited. To fulfill the criteria of curative treatment, they should either receive bridging stent placement with subsequent definitive colectomy, or emergency colectomy. The primary outcome was 3-year overall survival. Survival status of patients was traced till 30/5/19.
Results
70 (49M:21F) patients were recruited. The mean age was 64. The mean follow-up was 1173 days. 27 had bridging stent with 6 failed, all received emergency colectomies. 21 in stenting group received elective colectomies 24 weeks. 43 had emergency colectomy. Significantly more patients in stenting group had laparoscopic surgery for eventual colectomy (2.3% vs 63.0%, p<0.001). Significantly less patients in resection group received immediate bowel anastomosis (27.9% vs 70.4%, p<0.001). Amongst 12 patients who did receive immediate bowel anastomosis, 2 required stoma. The proportion of patients in resection group with stoma was significantly higher when compared with stenting group (76.7% vs 29.6%, p<0.001). 41 (58.6%) had stage III disease. 31(44.3%) had disease recurrence or metastasis, 22(31.4%) had peritoneal metastasis. Peritoneal metastasis amongst resection versus stenting group was 25.6% and 44.4% respectively (p=0.102). The overall 3-year survival was 63% vs 80%, p=0.369).
Conclusion
For patients with LSIO, bridging stent insertion and delayed colectomy showed no significant difference in oncological outcome in terms of peritoneal metastasis and three-year survival. With available expertise, bridging stent insertion and delayed colectomy is a safe alternative to emergency colectomy for treating patients with LSIO.
Keywords
Stenting; Bowel obstruction; Colorectal tumor.
Background
In patients with Intestinal Obstruction (IO) due to left sided colorectal malignancy, the conventional surgical options are resection of obstructing tumor or creation of defunctioning stoma. An alternative to emergency surgery is emergency Self- Expandable Metal Stent (SEMS) placement. If successful, this may achieve bowel decompression and allow definitive colectomy after sufficient bowel decompression. This can potentially convert a surgical emergency into a stable surgical condition. It allows subsequent operation to be performed in elective manner, which in turn promotes bowel anastomoses and lowers the need for stoma. Such benefits have already been proven in various studies.
However, the effect of such “bridging stent” insertion on long term oncological outcome remains unknown. With successful stent expansion, there is a theoretical risk of peritoneal spread due to tumor stretching and micro perforation by colonic stent. The results of previous studies are discordant over the issue of long term oncological safety. According to European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline, “bridging stent” is not recommended as a standard treatment for leftsided malignant colonic obstruction1. Therefore, we have reviewed the relevant data for our patients presented with IO due to left-sided colorectal malignancy. We hope to investigate the long term oncological outcome of “bridging stent” insertion for patient treated with a curative intent.
Methods
A retrospective review of patient record was performed. Patients who were admitted to Tuen Mun Hospital or Pok Oi Hospital between 1st January 2013 and 31st December 2017 with a diagnosis code of “Intestinal Obstruction (560.X)”, and with at least one subsequent operative record with diagnosis code of “Malignant Neoplasm of Splenic Flexure (153.7), Descending colon (153.2), Sigmoid colon (153.3), Rectosigmoid junction (154.0), Rectum (154.1), Other specified sites of large intestine (153.8), Colon unspecified (153.9), or Other sites of rectum, Rectosigmoid junction and anus (154.8)” were identified from Clinical Analysis and Data Reporting System (CDARS) of the Hospital Authority. The clinical records of all these patients were manually reviewed. The inclusion criteria for this review were patients with emergency admission due to IO caused by leftsided colorectal malignancy (i.e. obstructive tumour located between rectosigmoid junction and splenic flexure), patients with absence of distant metastasis upon diagnosis of IO, and patients who received curative treatment subsequently. To fulfil the criteria of curative treatment, they should either receive “bridging stent” placement with subsequent definitive colectomy, or emergency colectomy. In either case, the colectomies were performed in accordance with oncological standard (complete removal of colonic tumour with adequate margin by means of an adequate segmental resection, and corresponding lymphadenectomy with intent of sufficient lymph node harvest for nodal staging).
The selected patients were in fact surgical patients admitted with acute intestinal obstruction managed by general surgeons. Upon the request of attending surgeons, colorectal consultation for feasibility of SEMS placement could be issued. If SEMS placement was decided, it would be performed by colorectal surgeons under endoscopic and fluoroscopic guidance, followed by definitive colectomy after 2-4 weeks of bowel decompression. Otherwise, emergency colectomy would be performed by general surgeons on duty roster. They were grouped as stenting group if SEMS placement was ever attempted as the first curative treatment, and were grouped as resection group if emergency colectomy was offered instead.
For all selected patients, procedure and operative details were recorded, including failure of SEMS placement or related complications, subsequent bowel anastomosis or stoma, and leakage from anastomosis. Pathology of the subsequently resected specimens were also recorded. Record of subsequent adjuvant treatment was traced as well. The primary outcome was the three-year overall survival status of the patients. In addition, we also noted for peritoneal recurrence of patients as evidenced by surveillance imaging or subsequent operation. The survival status of patients was traced up till 30th May 2019.
Statistical analysis was performed using IBM SPSS Statistics version 26.0. Independent sample T-test was used for comparison of continuous variables. Pearson Chi-square or Fisher exact tests were used for comparison of categorical variable. For survival analysis, life table was employed. Comparison of survival curves were performed using log rank tests.
Results
Seventy patients were identified from CDARS and fulfilled the selection criteria. There were 49 males and 21 females. Their mean age was 64 years (35-84 years, standard deviation (SD)=11.6 years). They were all admitted with IO caused by leftsided colorectal malignancy, who eventually received curative treatment. The mean follow-up period was 1173 days (standard deviation (SD) 602.3 days).
After the diagnosis of IO due to left-sided colorectal malignancy, twenty-seven patients were assessed by colorectal surgeons and were offered bridging SEMS placement as their first treatment. The procedure of stent insertion failed in three patients due to technical difficulty. One patient was complicated with stent perforation, and 2 patients showed clinical failure after stent insertion. These six patients therefore received emergency colectomies as salvage. For the 21 patients who achieved in bowel decompression after SEMS placement, they received elective colectomies within 2-4 weeks from their stenting procedure. Altogether, these 27 patients with colonic stent insertion ever performed or attempted constituted the stenting group. For the remaining 43 patients who received emergency colectomy as their first treatment, they were grouped as resection group. The operations eventually performed for these 70 patients are listed in Table 1.
| Nature of Operation | Resection Group (n=43) | Stenting Group (n=27) |
|---|---|---|
| Extended right hemicolectomy (with primary anastomosis) | 2 | 0 |
| Extended right hemicolectomy (with exteriorization) | 1 | 0 |
| Lt hemicolectomy (with primary anastomosis) | 1 | 9 |
| Lt hemicolectomy (with exteriorization) | 3 | 1 |
| Hartmann’s procedure | 22 | 7 |
| Sigmoidectomy (with primary anastomosis) | 13 | 8 |
| Subtotal colectomy (with primary anastomosis) | 0 | 2 |
| Anterior resection (with primary anastomosis) | 1 | 0 |
Table 1: Nature of operation (colectomy).
A comparison of various baseline characteristics and procedure details between patients in resection group and stenting group is shown in Table 2. In terms of age and sex distribution, there is no difference between the two groups. There was a significantly higher proportion of patients in stenting group received laparoscopic surgery for their eventual colectomy (2.3% vs 63.0%, p<0.001). In addition, a significantly lower proportion of patients in resection group received immediate bowel anastomosis (27.9% vs 70.4%, p<0.001). Even among the 12 patients who did receive immediate bowel anastomosis, two of them required a covering stoma. Therefore, the proportion of patients in resection group ended up with stoma of any sort was significant higher when compared with stenting group (76.7% vs 29.6%, p<0.001).
| Resection Group (n=43) | Stenting Group (n=27) | p-value | |
|---|---|---|---|
| Age (SD) | 66 (12.0) | 63 (11.0) | 0.266+ |
| Male (%) | 30 (69.8%) | 19 (70.4%) | 0.957+ |
| Immediate anastomosis after bowel resection (%) | 12 (27.9%) | 19 (70.4%) | <0.001+ |
| anastomotic leakage (%) | 1 in 12 (8.3%) | 1 in 19 (5.3%) | 1.0000+ |
| End stoma or defunctioning stoma (%) | 33 (76.7%) | 8 (29.6%) | <0.001+ |
| Laparoscopic surgery (%) | 1 (2.3%) | 17 (63.0%) | <0.001+ |
Table 2: Baseline characteristics and procedure details.
*Independent sample T-tests, +Pearson Chi-square or Fisher exact tests.
The pathological details of resected colonic tumour are shown in Table 3. There were 41 patients (58.6%) diagnosed with stage III disease. Comparison between resection group and stenting group of patients showed no significant difference in percentage of pathological T4 patients or stage III patients. Except for the three patients who died within 30 days from their operations, all patients were referred for oncology assessment for consideration adjuvant chemotherapy. Thirty-three patients either declined or were deemed unfit for adjuvant treatment. Thirty-four patients eventually went through adjuvant treatment. There was no significant difference in percentage of patient received adjuvant chemotherapy between both groups.
| Pathology of Resected Colonic Tumour | Resection Group (n=43) | Stenting Group (n=27) | p-value |
|---|---|---|---|
| pT4 (%) | 8 (18.6%) | 7 (25.9%) | 0.467+ |
| pT3 (%) | 35 (81.4%) | 19 (70.4%) | 0.285+ |
| pT2 (%) | 0 (0%) | 1 (3.7%) | 0.386+ |
| Stage III (%) | 28 (65.1%) | 13 (48.1%) | 0.161+ |
| Adjuvant chemotherapy (%) | 19 (44.2%) | 15 (55.6%) | 0.354+ |
Table 3: Pathological Details.
+Pearson Chi-square or Fisher exact tests.
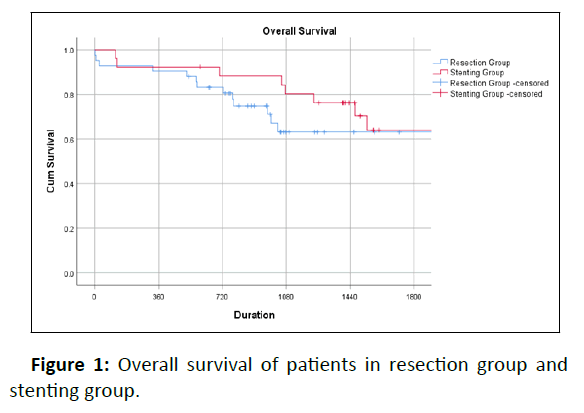
During post-operative surveillance, thirty-one patients (44.3%) were eventually diagnosed with disease recurrence or metastasis, with 22 (31.4%) having peritoneal metastasis. The percentage of patients with peritoneal metastasis among resection versus stenting group was 25.6% and 44.4% respectively (p=0.102). The overall 3-year survival for these two groups of patients was 63% vs 80% (log-rank tests, p=0.369) (Figure 1).
Discussion
In patient presented with colonic obstruction due to left-sided colorectal tumour, the primary advantage of emergency SEMS placement derives from its potential in achieving bowel decompression through stenting, thereby converting an emergency colectomy into an elective one. This may in turn increase the chance of bowel anastomosis after colectomy and prevent stoma creation. The findings of this study confirms a higher rate of bowel anastomosis and lower chance of stoma with the use of “bridging stent” insertion, with no significant increase in anastomotic leakage when compared with emergency resection. This is in line with reports from other studies [1].
The oncological safety of emergency SEMS placement has remained controversial. The long term follow-up of prospective randomized controlled trial from a local group showed that the overall and 5-year disease-free survival of endolaparoscopic group (patients were treated by bridging stent followed by elective laparoscopic colectomy) was comparable with open surgery group (patient were treated with emergency open colectomy) [2]. Similarly no significant difference between stenting group and resection group in terms of overall recurrence [3]. In contrast, a significantly lower overall 5-year survival for SEMS group (30%) when compared with surgery group (30% vs 67%, p=0.001), even after patients with perforation or metastasis were excluded from analysis [4]. In Stent-in 2 Trial, the authors have found an association between stent perforation and tumor recurrence [5]. However, due to relatively small number of patients in subgroup analysis, the authors failed to conclude on the causation of tumor recurrence by stent perforation.
In order to investigate the effect of SEMS placement (and its complications) with long term oncological outcome of patients, instead of grouping our patients according to the timing of operation (elective or emergency), we have grouped our patients according to the performance of colonic stenting. In post-op surveillance, we have found no significant difference in peritoneal metastasis and overall 3-year survival between the stenting and resection group of patients. Even after inclusion of patients with stent perforation, the result of our patients who were treated with bridging stent was comparable with those who were treated primarily with resection.
There are several limitations in this study. The retrospective nature of our study has suffered from inherent selection bias. Our search strategy has helped us to identify patients who received curative treatment to their obstructing left-sided colorectal tumor. However, we may have omitted those with the intent of treatment unclear upon diagnosis. There may also be additional omission due to incorrect or incomplete diagnosis coding. With all SEMS placement performed by colorectal surgeons, who regularly perform nearly 20 colorectal stenting every year, our center is indeed providing a highly subspecialized colorectal service supporting the emergency surgery. This may not be practical to all surgical centers in Hong Kong.
For patients with IO due to left-sided colorectal malignancy, bridging stent insertion and delayed colectomy may achieve less disruption of bowel continuity then emergency colectomy. There is no significant difference between these two options in terms of oncological outcome including peritoneal metastasis and three-year survival. With available expertise and skills, bridging stent insertion and delayed colectomy is a safe alternative to emergency colectomy for treating patients with IO due to leftsided colorectal malignancy.
Reference
- Van HJE, Van HEE, Vanbiervliet G, Beets-Tan RG, DeWitt JM et. al (2014) Self-expandable metal stents for obstructing colonic and extracolonic cancer: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Gastrointest Endosc 80: 747-761.
[Crossref], [Google Scholar], [Indexed]
- Tung KLM, Cheung HYS, Ng LWC, Chung CCC, Li MKW (2013) endo-laparoscopic approach versus conventional open surgery in the treatment of obstructing left-sided colon cancer: Long-term Follow-up of a randomized trial. Asian J Endosc Surg 6: 78-81.
[Crossref], [Google Scholar], [Indexed]
- Alcantara M, Serra AX, Falco J, Mora L, Bombardo J et. al (2011) Prospective, controlled, randomized study of intraoperative colonic lavage versus stent placement in obstructive left-sided colonic cancer. World J Surg 35: 1904-10.
[Crossref], [Google Scholar], [Indexed]
- Sabbagh C, Browet F, Diouf M, Cosse C, Brehant O et. al (2013) Is stenting as "a bridge to surgery" an oncologically safe strategy for the management of acute, left-sided, malignant, colonic obstruction? A comparative study with a propensity score analysis. Ann Surg 258: 107-15.
[Crossref], [Google Scholar], [Indexed]
- Sloothaak DA, Van den BMW, Dijkgraaf MG, Fockens P, Tanis PJ et. al (2014) Oncological outcome of malignant colonic obstruction in the Dutch stent-In 2 trial. Br J Surg 101: 1751-7.
[Crossref], [Google Scholar], [Indexed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences