Interplay between Gut Microbiota and T Lymphocytes in Colorectal Cancer
Helal F Hetta*, Azza Elkady, Mohamed A Mekky, Mohamed O Abdelmalek, Hani I Sayed, Shamardan E Bazeed and Khairy H Morsy
DOI10.21767/2471-9943.100042
Helal F Hetta1*, Azza Elkady2, Mohamed A Mekky3, Mohamed O Abdelmalek3, Hani I Sayed4, Shamardan E Bazeed5 and Khairy H Morsy6
1Department of Medical Microbiology and Immunology, Faculty of Medicine, Assiut University, Assiut, Egypt
2Sohag General Hospital, Sohag University, Sohag, Egypt
3Department of Tropical Medicine and Gastroenterology, Assiut University Hospital, Assiut, Egypt
4Center for Management of Viral Hepatitis, Ministry of Health, Assiut, Egypt
5Department of Tropical Medicine and Gastroenterology, South Valley University, Qena University Hospital, Qena, Egypt
6Department of Tropical Medicine and Gastroenterology, Faculty of Medicine, Sohag University, Sohag, Egypt
- *Corresponding Author:
- Helal F Hetta
Department of Medical Microbiology and Immunology, Faculty of Medicine, Assiut University, Assiut, Egypt 71515
Tel: 002 01002386255
E-mail: helalhetta@asun.edu.eg (or) helalhetta@yahoo.com
Received date: July 04, 2017; Accepted date: July 29, 2017; Published date: August 04, 2017
Citation: Helal FH, Azza E, Mohamed AM, Mohamed OA, Hani IS, et al. (2017) Interplay between Gut Microbiota and T Lymphocytes in Colorectal Cancer. Colorec Cancer. Vol.3 No.2:12. doi: 10.21767/2471-9943.100042
Copyright: © 2017 Helal FH, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Colorectal cancer is one of the third leading causes of cancer mortality worldwide. There is increasing evidences about the involvement of gut microbiota in colorectal carcinogenesis. Several recent studies have shed light on the cross talk between gut microbiota and mucosal T cell subsets, specifically intraepithelial lymphocytes and lamina propria CD4+ T cells including T-helper cells and Tregulatory (Treg) cells which may be responsible for development of colorectal cancer through creating imbalance between proinflammatory and antiinflammatory immune cells and their cytokines. In this review, we will focus on the influence gut microbiota on the development of mucosal T cells and their role in promoting colorectal cancer.
Keywords
Gut microbiota; CRC; T lymphocytes
Introduction
Colorectal cancer (CRC) is one of the most common public health burden. It is the third leading cause of cancer worldwide after lung and prostate, with nearly 600,000 death each year [1].
Most of colorectal cancers originate from epithelial cells of the colorectal mucosa, being identified by the formation of glandular structures and histologically classified as adenocarcinomas. The carcinogenesis process of CRC includes four main phases; initiation, promotion, progression and metastasis [2,3].
CRC is usually associated with alteration in gut microbiota composition and shift in distribution of bacterial communities. However, these studies neither answer the cause or consequence question of dysbiosis in CRC, nor do they provide mechanistic insights by which the intestinal microbiota influences the development of CRC [4]. Also, several studies found that gut microbiota have a strong impact on regulation and development of immune system which could in turn initiate regulation of immune cells within the lamina propria of the intestine as well as mediating the inflammatory process [5].
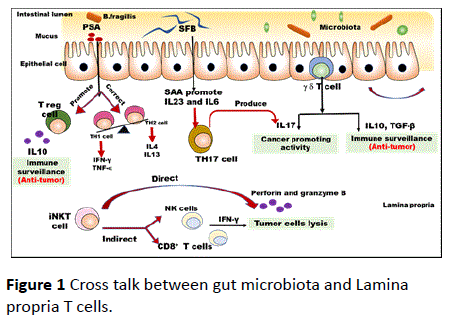
In this review, we will focus on how gut microbiotas could regulate the development mucosal T cell subsets, specifically intraepithelial lymphocytes and LP CD4+ T cells including Thelper cells and Treg cells. The result of the cross talk between gut microbiota and different T lymphocytes is the imbalance between proinflammatory and inflammatory immune response which in turn will provide suggested mechanisms of promoting colorectal cancer as shown in Figure 1.
PSA (polysaccharide A) is a symbiosis factor that promotes antitumor activity by enhancement of development of regulatory T cells to produce IL10 and correct imbalance of Thelper 2 toward T-helper 1 which produce IFN-gamma and TNF-α.
On the other hand, segmented filamentous bacteria (SFB) enhance production of serum amyloid A (SAA) which promote Th17 development which has cancer promoting activity. (IEL) intraepithelial lymphocytes (γδ T cells) produce IL10 and TGF-β which has antitumor activity, on the other hand it can also produce IL17 which promote cancer activity, suggesting the two-opposite role of (IEL) in colorectal cancer. Invariant natural killer T (iNKT) cells can recognize endogenous lipids presented by CD1d molecules on tumor cells and subsequently eliminate tumor cells directly through iNKT cell-mediated lysis. In the absence of CD1d expression on tumor cells, iNKT cells mediate tumor cell lysis indirectly through activation of NK cells and CD8+ tumor specific T- cells.
Gut microbiota and TH1-TH2 axis
TH1 CD4+ T lymphocytes produce cytokines IL-2, interferon (IFN)-gamma and tumor necrosis factor (TNF)-beta which in turn activate macrophages and responsible for cell mediate immune response. On the other hand, humoral immunity and antibody production are strongly associated with TH2 CD4+ T lymphocytes producing cytokines including IL-4, IL-6 and IL-10 [6]. Immune deviation towards TH1 anti-inflammatory responses results in tumor rejection as TH1 pathways are responsible for cytotoxic T-cell lymphocytes (CTL), natural killer (NK) cells, macrophages and monocytes, all of which can attack cancer cells and generally defend against tumors [6,7]. Conversely deviation towards TH2 type inflammation response promote tumor growth [8,9]. Almost all malignancies are associated with suppression of the cell mediated immunity. Several studies have reported that shift in immune function has been demonstrated in patients with colorectal cancer [10-12]. The numbers of TH1 CD4+ cells were decreased in patients with colorectal tumors, with low production of cytokines from TH1 lymphocytes whilst there was elevation in the levels of cytokines produced by TH2 lymphocytes [13,14]. The further the disease process has progressed the more significant these imbalances become with high levels of the humoral immunity associated cytokines having a prognostic influence in terms of disease-free survival and tumor recurrence post-surgery [15].
Interactions between lamina propria CD4+ T cells and the gut microbiota, often with epithelial cells or dendritic cells functioning as the intermediaries, are critical for shaping the adaptive immune response in the intestine. As bacterial colonization of the intestine is considered to be one of the most important factor for mammalian immune system function. It has been found that B. fragilis was the first identified bacterial species which are sufficient enough to correct the Th1 and Th2 cell imbalances observed in non-gut lymphoid tissues in germ free mice. B. fragilis is a Gramnegative anaerobic symbiont which colonize 30-70% of human lower intestinal tract as primarily reservoir [16,17]. Colonization of GF mice with B. fragilis induced overall CD4+ T cell expansion and increased numbers of Th1 cells to levels similar to those of conventionally raised mice. Mechanistically, B. fragilis is mainly exert their function through production of polysaccharide A (PSA) [17,18]. Among carbohydrates, PSA is known to be potent T cell–dependent rather than a T cellindependent antigen. Also, it is the most effective T-cell dependent antigen that can strongly regulate host immune system [17].
It has been found that PSA produced by B. fragilis in germ free mice, can induce Th1 expansion and cytokine production. Th1 development is mainly depend on IL12 which are produced by activated dendritic cells. PSA is the most effective polysaccharide known to signal dendritic cells to induce IL-12 production [19].
Gut microbiota and Th17
Several recent studies tried to focus on how the commensal bacteria affects the development of Th17 cells and their in promoting CRC. Th17 cells have been implicated in the pathogenesis of several human autoimmune diseases and inflammatory disease models via proinflammatory cytokines production [20-23]. On the other hand, Th17 cells play an important role in host defense against extracellular bacteria and parasites [24]. Several animal studies have found that Th17 cells which are mainly localized in the lamina propria of the small intestine and the colon and play a critical role in defense against Shigella flexneri and Citrobacter rodentium [25,26].
Although, it was found that segmented filamentous bacteria (SFB) factors lead to increase number of TH17 cells, many questions still remain about how SFB drive gut Th17 cell development. Which provide a suggested link between gut microbiota and Th17 cells. SFB adhesion to the epithelium induces Th17 development via production of serum amyloid A (SAA) which in turn induced dendritic cells to produce IL-6 and IL-23, the key cytokines for Th17 development which are contributed to progression of intestinal inflammation [24] as shown in Figure 1.
IL-17, pleiotropic proinflammatory cytokine which are highly produced in the tumor microenvironment, plays important roles in both angiogenesis and tumor immunity. Accumulative evidences have suggested that IL17 expression in elevated in many human tumors such as breast, ovarian, hepatocellular and CRC [27]. Consistently with a positive role of IL-17 in promoting tumor development, tumor tissues have a higher frequency of IL-17+ cells T cells compared with untransformed bowel tissues [28]. The protumor role of IL-17 which is mediated by inflammation-associated signaling pathways, provides additional support for the already well-established link between inflammation and tumorigenesis [29]. It has been proposed that enterotoxin producing Bacteroides fragilis (ETBF) human, colonic commensal bacterium, are able to promote colonic tumorigenesis in APC mutant mice through activation of Stat3 pathway and Th17-cell polarization and the block of IL23 R and IL17 could reduce the tumor formation [30]. It has been reported that worsen of intestinal epithelial barrier result in microbial pathogen invasion and microbial products release are driving IL-23/IL-17 axis activation and promoting tumor growth and progression in CPC-APC mice [31].
Gut microbiota and regulatory T-cells
Although, Treg cells are one of the key players for maintaining intestinal homeostasis, immune surveillance and immune therapy of cancer due to suppressing anti-tumor immune responses, several studies using Apc Min/+ mice revealed a protective role of IL-10-producing Treg cells in bacterial-induced chronic inflammation and cancer [32] and even hereditary colon cancer, suggesting that Treg cells might play a protective role in cancer by suppressing inflammation [33].
The gut contains a high frequency of Treg cells which comprise two main subtypes, naturally occurring Tregs that develop in the thymus before trafficking to other sites in the body and inducible Tregs that develop in the periphery [34-40]. Foxp3+ intestinal Tregs are mainly localized in the lamina propria of the small intestine and colon [41] and according to these findings, it was implied that accumulation of Tregs in the colon and SI is differentially regulated and the colonic Treg differentiation is more heavily influenced by the microbiota [41]. Several results among mouse models of colitis have shown that disruption of regulatory T-cell networks can initiate chronic intestinal inflammation in the presence of a triggering intestinal microbiota [42].
It was important to demonstrate how single organism and defined bacterial communities can instruct Treg responses. Recent works provide our knowledge with the answers to these questions, as they show that B. fragilis and its immunomodulatory molecule, PSA, are capable of driving Treg differentiation [43]. PSA derived from the human commensal Bacteroides fragilis is a symbiosis factor that stimulates immunologic development within mammalian hosts. One of the most important function of PSA is enhancement of suppressive function of circulating Foxp3(+) Tregs [44,45].
Intraepithelial Lymphocytes (IELs)
IELs are specialized T cells that are consisting of two major groups depending on expression of T-cell receptor-γδ (TCRγδ) or TCRαβ, also can be divided into two populations, including natural and induced IELs. In human, IELs, are mainly found in direct contact with the enterocytes, to exert their critical role in the maintenance of intestinal homeostasis [46]. Because of the specific location of IELs in intestinal mucosa, they are involved in resisting to the invasion of foreign pathogens and preserving immune regulation. It has been found that TCRγδ+ IELs perform their defense mechanism against intestinal pathogens via secretion of IFN-γ and TNF, and importantly to drive proinflammatory effects [47,48]. TCRγδ+T cells could increase the production of TGF-β and IL-10 to perform their suppressive function in intestinal inflammation and so immune regulation of intestinal mucosa [49,50].
In the first study on effect of regulatory role of gamma-delta T cells in immunopathogensis of colorectal cancer, they found that the imbalance e in Vδ1 and Vδ2 T cells creates an immunosuppressant microenvironment in rectal cancer tissues, which may enable tumors to limit antitumor immunity and evade immune surveillance in rectal cancer patients [51].
Recently, a protumor role for IL-17-producing γδ T cells was reported for the first time in human cancer. Specifically, Vδ1+T cells were the major source of IL-17 involved in chronic inflammation in colorectal cancer. Moreover, IL-17-producing γδ T cells secreted IL-8, tumour necrosis factor (TNF) and granulocyte–macrophage
Colony-stimulating factor (GM-CSF), which recruited immunosuppressive MDSCs into the malignant microenvironment, further driving protumour inflammation. Importantly, the extent of IL-17-producing γδ T cell
Importantly, the extent of IL-17-producing γδ T cell infiltration positively correlated with the clinical stage of the disease, highlighting the potential cancer-promoting role of IL-17-producing γδ T cells in human colorectal cancer [52].
Gut microbiota and CD1d+, NKT cells
Unlike T-cells, NKT cells are initially recognized to co-express T cell and natural killer cell (NK cell) markers (hence, the name NKT cells) and later defined by their ability to recognize self and non-self-lipid antigens presented by the atypical MHC class I molecule CD1d, which is required for NKT cell positive selection in the thymus [53]. Classification of NKT cells into invariant (i) or type I NKT cells and non-invariant or type II NKT cell is mainly depend on the expression of T- cell receptor (TCR).
Importantly, it has been found that intestinal microbiota exerts their regulatory effect through CD1d-iNKT cell. Thus, in addition to microbiota-dependent control of intestinal iNKT cells, CD1d and NKT cells regulate intestinal microbial colonization and the composition of the microbiota [54]. Moreover, CD1d deficiency is associated with intestinal dysbiosis in accordance with the regulation of the intestinal microbiota by CD1d and NKT cells. Similarly, CD1d-deficient mice are susceptible to wide variety of mucosal pathogens [55]. Collectively, mucosal iNKT cells and the intestinal microbiota cross-regulate each other in a bidirectional manner.
In cancer, although few number of clinical trials in have been performed in human to investigate its role in cancer, there was a strong evidence for the role of type I NKT cells are predominantly protective, helping to orchestrate CD8+ T cells and NK cells to inhibit tumor growth through their production of interferon-γ, and through their activation of dendritic cells to make IL-12 and other cytokines [56]. Conversely, we have seen that type II NKT cells are the primary type of NKT cells responsible for suppression of tumor immunity, through their ability to make IL-13 and induce production of TGF-β by myeloid cells [57]. Indeed, it is becoming increasingly recognized that one of the important sources of the TGF-β that suppresses tumor immunity may be the immune system itself, not just the tumor, and this may be largely through this mechanism of type II NKT cell induction or other mechanisms that trigger TGF-β production by myeloid cells. IL-13 from NKT cells can also recruit M2 tumor-associated macrophages that can suppress tumor immunity [58,59].
A recent study was done on a mouse colitis-associated colorectal cancer model reported that a significant decrease in iNKT cells. The deficiency of iNKT cells were associated with increased in numbers of tumors and resulted in more severe colitis in an AOM ⁄DSS colorectal cancer model. Furthermore, activation of iNKT cells by GC resulted in a decrease in the incidence of tumors in this model, probably due to the inhibition of IL-13-producing NK1.1+ T cells. These findings provide support for the hypothesis that iNKT cells are implied in protection against tumors in colitis-associated cancer [60].
Upon activation with a-GC, iNKT cells display powerful antitumor cytotoxic activity [61], rapidly release cytokines (such as GM-CSF, TNF-a, IFN-g, IL-4, IL-10 and IL-13 [62,63] and promote maturation of dendritic cells into APC that are capable of activating conventional T cells [64].
Further work is required to determine the regulation of mucosal NKT cells frequency, and function of human colorectal cancer.
Conclusion
There is a growing awareness of the importance of gut microbiota in mediating colorectal cancer as it’s the third leading cause of cancer all over the world. However, it remains questionable how the cross talk between gut microbiota and laminal CD4 T-cells and intraepithelial lymphocytes could influence on their involvement in colorectal malignancy. Herein this review we have provided a different suggested role in the colorectal carcinogenesis. Commensal gut microbiota could have antitumor activity via gamma IFN and TNF producing CD4+ TH1 cells and IL10 secreted by FoxP3+ regulatory T cells. Invariant natural killer T (iNKT) cells, which are naturally found in intestine, can recognize endogenous lipids presented by CD1d molecules on tumor cells and subsequently eliminate tumor cells directly through iNKT cellmediated lysis. In the absence of CD1d expression on tumor cells, iNKT cells mediate tumor cell lysis indirectly through activation of NK cells and CD8+ tumor specific T- cells. γδ T cells are intraepithelial lymphocytes have produce IL10 and TGF-β which has antitumor activity. On the other hand, some segmented filamentous bacteria (SFB) drive Th17 development which has cancer promoting activity via production of IL17. Further prospects for better managements of colorectal cancer aim to metagenomics and metatranscriptomic surveys of the microbiota which in turn will provide our knowledge with information about the membership and function of the microbial world within the gut.
References
- Haggar FA and Boushey RP (2009) Colorectal Cancer Epidemiology: Incidence, Mortality, Survival, and Risk Factors. Clin Colon Rectal Surg 22: 191-197.
- Matthew F, Sreelakshmi R, Sergei FT, Hanlin LW (2012) Colorectal carcinoma: Pathologic aspects. J Gastrointest Oncol 3: 153-173.
- Ponz de Leon M, Percesepe A (2000) Pathogenesis of colorectal cancer. Dig Liver Dis 32: 807-821.
- Marchesi JR, Dutilh BE, Hall N, Peters WH, Roelofs R, et al. (2011) Towards the human colorectal cancer microbiome. PLoS One 6: e20447.
- Irrazábal T, Belcheva A, Girardin SE, Martin A, Philpott DJ, et al. (2014) The Multifaceted Role of the Intestinal Microbiota in Colon Cancer. Molecular Cell. 54: 309-320.
- Loose D and Van de Wiele C (2009) The immune system and cancer. Cancer Biother Radiopharm 24: 369-376.
- Kidd P (2003) Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Alternative medicine review 8: 223-246.
- Terabe M, Park JM, Berzofsky JA (2004) Role of IL-13 in regulation of anti-tumor immunity and tumor growth. Cancer Immunology, Immunotherapy 53: 79-85.
- Romagnani S (1997) The th1/th2 paradigm. Immunology today 18: 263-266.
- Monteleone G, Pallone F, Stolfi C (2012) The dual role of inflammation in colon carcinogenesis. Int J Mol Sci 13: 11071-11084.
- Evans C, Morrison I, Heriot AG, Bartlett JB, Finlayson C, et al. (2006) The correlation between colorectal cancer rates of proliferation and apoptosis and systemic cytokine levels; plus, their influence upon survival. British journal of cancer. 94: 1412-1419.
- Grivennikov SI (2010) Immunity, Inflammation, and Cancer. 140: 883-899.
- Nakayama H, Kitayama J, Muto T, Nagawa H et al. (2000) Characterization of intracellular cytokine profile of CD4 (+) T cells in peripheral blood and tumor-draining lymph nodes of patients with gastrointestinal cancer. Japanese journal of clinical oncology 30: 301-305.
- Kanazawa M, Yoshihara K, Abe H, Iwadate M, Watanabe K, et al. (2005) Effects of PSK on T and dendritic cells differentiation in gastric or colorectal cancer patients. Anticancer research 25: 443-449.
- Shibata M, Nezu T, Kanou H, Abe H, Takekawa M, et al. (2002) Decreased production of interleukin-12 and type 2 immune responses are marked in cachectic patients with colorectal and gastric cancer. J Clin Gastroenterol 34: 416-420.
- Sears CL and Pardoll DM (2011) Perspective: alpha-bugs, their microbial partners, and the link to colon cancer. J Infect Dis 203: 306-311.
- Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL, et al. (2005) An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 122: 107-118.
- Michael JC, Arthur OT, Benjamin CM, Vincent JC, Dennis LK, et al. (2001) Polysaccharide biosynthesis locus required for virulence of Bacteroides fragilis. Infect Immun 69: 4342-4350.
- Wang Q, McLoughlin RM, Cobb BA, Charrel-Dennis M, Zaleski KJ, et al. (2006) A bacterial carbohydrate links innate and adaptive responses through Toll-like receptor 2. J Exp Med 203: 2853-2863.
- Helal F. Hetta, Azza Elkady, Khairy H. Morsy, Ismail S Mohamed, Khaled M. Hassanein, et al. (2017) Circulating IL17a and IFN-Gamma Serum Levels in Cirrhotic Hepatitis C Virus Infected Patients with Autoimmune Thyroiditis. International Journal of Current Microbiology and Applied Sciences 6: 1972-1983.
- Hirota K, Hashimoto M, Yoshitomi H, Tanaka S, Nomura T, et al. (2007) T cell self-reactivity forms a cytokine milieu for spontaneous development of IL-17+ Th cells that cause autoimmune arthritis. Journal of Experimental Medicine 204: 41-47.
- Henriques A, Inês L, Couto M, Pedreiro S, Santos C, et al. (2010) Frequency and functional activity of Th17, Tc17 and other T-cell subsets in Systemic Lupus Erythematosus. Cell Immunol 264: 97-103.
- Helal FH, Azza Elkady, Khairy HM, Ismael SM (2017) Serum Level of IL17a among Cirrhotic Hepatitis C Virus Infected Patients with Incidence of Diabetes Mellitus. The Egyptian Journal of Immunology 24.
- Korn T, Bettelli E, Oukka M, Kuchroo VK, et al. (2009) IL-17 and Th17 Cells. Annu Rev Immunol 27: 485-517.
- Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, et al. (2009) Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139: 485-498.
- Sellge G, Magalhaes JG, Konradt C, Fritz JH, Salgado-Pabon W, et al. (2010) Th17 Cells Are the Dominant T Cell Subtype Primed by Shigella flexneri mediating protective immunity. The Journal of Immunology 184: 2076-2085.
- Helal F Hetta, Azza Elkady, Tohamy A Tohamy, Badary MS (2016) Regulatory B Cells: Key Players in Hepatocellular Carcinoma Progression. Gastroenterology & Hepatology: Open Access 5: 00136.
- Wook-Jin Chaea, Thomas F. Gibsona, Daniel Zeltermanb, Liming Haoc, Octavian Henegariu, et al. (2010) Ablation of IL-17A abrogates progression of spontaneous intestinal tumorigenesis. Proc Natl Acad Sci USA 107: 5540-5544.
- Girardin A, McCall J, Black MA, Edwards F, Phillips V, et al. (2013) Inflammatory and regulatory T cells contribute to a unique immune microenvironment in tumor tissue of colorectal cancer patients. Int J Cancer 132: 1842-1850.
- Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, et al. (2009) A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nature medicine 15: 1016-1022.
- Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, et al. (2012) Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature 49: 254-258.
- Erdman SE, Rao VP, Poutahidis T, Ihrig MM, Ge Z, et al. (2003) CD4(+)CD25(+) regulatory lymphocytes require interleukin 10 to interrupt colon carcinogenesis in mice. Cancer Res 63: 6042-50.
- Erdman SE, Sohn JJ, Rao VP, Nambiar PR, Ge Z, et al. (2005) CD4+CD25+ regulatory lymphocytes induce regression of intestinal tumors in ApcMin/+ mice. Cancer Res 65: 3998-4004.
- Hetta HF (2014) Gut immune response in the presence of hepatitis C virus infection. World Journal of Immunology 4: 52.
- Hetta HF, Mekky MA, Khalil NK, Mohamed WA, El-Feky MA, et al. (2015) Association of colonic regulatory T cells with hepatitis C virus pathogenesis and liver pathology. J Gastroenterol Hepatol 30: 1543-51.
- Hetta HF, Mekky MA, Khalil NK, Mohamed WA, El-Feky MA, et al. (2016) Extra-hepatic infection of hepatitis C virus in the colon tissue and its relationship with hepatitis C virus pathogenesis. J Med Microbiol 65: 703-712.
- Mehta M, Hetta HF, Abdel-Hameed EA, Rouster SD, Hossain M, et al. (2016) Association between IL28B rs12979860 single nucleotide polymorphism and the frequency of colonic Treg in chronically HCV-infected patients. Arch Virol 161: 3161-3169.
- Minesh Mehta, Helal Hetta, Mohamed Tarek, M. Shata et al. (2014) Sa1311 Significant Correlations Between the Frequency of Colonic Mucosal T Regulatory Cells and Liver Inflammation Using Metavir but Not APRI Scores in HCV-Infected Patients. Gastroenterology 146: S-259-S-260.
- Feuerer M, Hill JA, Mathis D, Benoist C (2009) Foxp3+ regulatory T cells: differentiation, specification, subphenotypes. Nat Immunol 10: 689-695.
- Helal FH, Mohamed AM, Nasr KK, Wegdan AM, Mohamed EL-Feky A, et al. (2013) Significant correlations between the frequency of colonic mucosal Treg cells with the viral load and liver inflammation in HCV-infected patients. Hepatology 58: 1182A-1182A.
- Atarashi K, Y. Umesaki, Honda K (2011) Microbiotal influence on T cell subset development. Semin Immunol 23: 146-53.
- Izcue A, Coombes JL, Powrie F (2009) Regulatory lymphocytes and intestinal inflammation. Annu Rev Immunol 27: 313-338.
- Round JL and Mazmanian SK (2010) Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci USA 107: 12204-12209.
- Telesford KM, Yan W, Ochoa-Reparaz J, Pant A, Kircher C, Christy MA, et al. (2015) A commensal symbiotic factor derived from Bacteroides fragilis promotes human CD39(+)Foxp3(+) T cells and Treg function. Gut Microbes 6: 234-242.
- Mazmanian SK, Round JL, Kasper DL (2008) A microbial symbiosis factor prevents intestinal inflammatory disease. Nature 453: 620-625.
- Cheroutre H, Lambolez F, Mucida D (2011) The light and dark sides of intestinal intraepithelial lymphocytes. Nat Rev Immunol 11: 445-56.
- Simpson SJ, Holländer GA, Mizoguchi E, Allen D, Bhan AK, et al. (1997) Expression of pro-inflammatory cytokines by TCR alpha beta+ and TCR gamma delta+ T cells in an experimental model of colitis. Eur J Immunol 27: 17-25.
- Sheridan BS and Lefrançois L (2010) Intraepithelial Lymphocytes: To Serve and Protect. Curr Gastroenterol Rep 12: 513-521.
- Inagaki-Ohara K, Chinen T, Matsuzaki G, Sasaki A, Sakamoto Y, et al. (2004) Mucosal T cells bearing TCRgammadelta play a protective role in intestinal inflammation. J Immunol 173: 1390-1398.
- Kühl AA, Pawlowski NN, Grollich K, Blessenohl M, Westermann J, et al. (2009) Human peripheral gammadelta T cells possess regulatory potential. Immunology. 128: 580-588.
- Rong L, Li K, Li R, Liu HM, Sun R, et al. (2016) Analysis of tumor-infiltrating gamma delta T cells in rectal cancer. World J Gastroenterol 22: 3573-3580.
- Wu P, Wu D, Ni C, Ye J, Chen W et al. (2014) gammadeltaT17 cells promote the accumulation and expansion of myeloid-derived suppressor cells in human colorectal cancer. Immunity 40: 785-800.
- Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L (2004) NKT cells: what's in a name? Nat Rev Immunol 4: 231-237.
- Nieuwenhuis EE, Matsumoto T, Lindenbergh D, Willemsen R, Kaser A, et al. (2009) Cd1d-dependent regulation of bacterial colonization in the intestine of mice. J Clin Invest 119: 1241-1250.
- Tupin E, Kinjo Y, Kronenberg M (2007) The unique role of natural killer T cells in the response to microorganisms. Nat Rev Microbiol 5: 405-417.
- van der Vliet HJ, Molling JW, Nishi N, Masterson AJ, Kölgen W, et al. (2003) Polarization of Valpha24+ Vbeta11+ natural killer T cells of healthy volunteers and cancer patients using alpha-galactosylceramide-loaded and environmentally instructed dendritic cells. Cancer Res 63: 4101-4106.
- Ambrosino E, Terabe M, Halder RC, Peng J, Takaku S. et al. (2007) Cross-regulation between type I and type II NKT cells in regulating tumor immunity: a new immunoregulatory axis. J Immunol 179: 5126-5136.
- Berzofsky JA and Terabe M (2008) NKT cells in tumor immunity: opposing subsets define a new immunoregulatory axis. J Immunol 180: 3627-3635.
- Terabe M, Matsui S, Noben-Trauth N, Chen H et al. (2000) NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nat Immunol 1: 515-520.
- Yoshioka K, Ueno Y, Tanaka S, Nagai K, Onitake T, et al. (2012) Role of Natural Killer T Cells in the Mouse ColitisÃÆâÃâââ¬ÃâÃÂAssociated Colon Cancer Model. Scandinavian journal of immunology,. 75: 16-26.
- Metelitsa LS, Naidenko OV, Kant A, Wu HW, Loza MJ, et al. (2001) Human NKT cells mediate antitumor cytotoxicity directly by recognizing target cell CD1d with bound ligand or indirectly by producing IL-2 to activate NK cells. The Journal of Immunology 167: 3114-3122.
- Mark Exley, Jorge Garcia, Steven P. Balk, Steven Porcelli (1997) Requirements for CD1d Recognition by Human Invariant. J Exp Med 186: 109-120.
- Gumperz JE, Miyake S, Yamamura T, Brenner MB et al. (2002) Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. Journal of Experimental Medicine 195: 625-636.
- Lynch L, O'Shea D, Winter DC, Geoghegan J, Doherty DG et al. (2009) Invariant NKT cells and CD1d+ cells amass in human omentum and are depleted in patients with cancer and obesity. European journal of immunology 39: 1893-1901.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences