Isolated Cerebellar Metastasis as the Initial Manifestation of Right-Sided Colon Cancer: A Case Report and Review of Current Management Strategies
Brandt L Esplin and Joleen Hubbard
DOI10.21767/2471-9943.100025
Division of Medical Oncology, Mayo Clinic, Rochester, MN 55905, United States of America
- *Corresponding Author:
- Joleen Hubbard
Division of Medical Oncology, Mayo Clinic, Rochester, MN 55905, United States of America.
Tel: 5072848318
Fax: 5075386133
E-mail: Hubbard.Joleen@Mayo.edu
Received date: August 25, 2016; Accepted date: August 31, 2016; Published date: Sepetember 06, 2016
Citation: Esplin BL, Hubbard J. Isolated Cerebellar Metastasis as the Initial Manifestation of Right-Sided Colon Cancer: A Case Report and Review of Current Management Strategies. Colorec Cancer 2016, 2:3.
Introduction
A 55 year old female with no significant past medical history presented to her local physician with a 2-week history of right hand incoordination and progressive gait instability. She had no family history of malignancy, and denied weight loss, appetite change, nausea, fatigue, abdominal pain, weakness, fevers, chills, or night sweats.
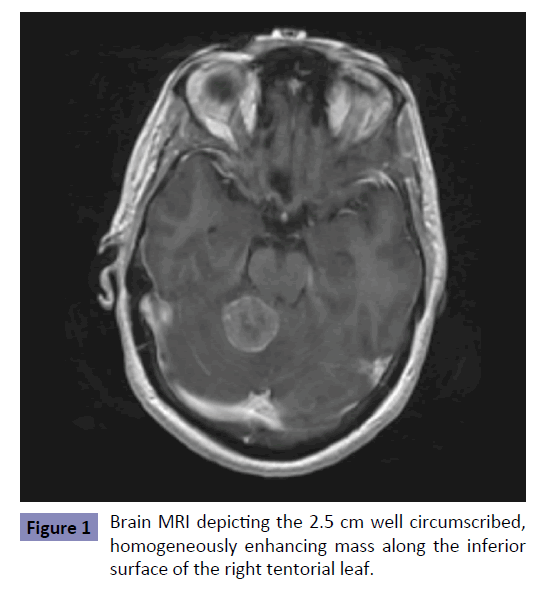
An MRI of her brain revealed an extra-axial-appearing cerebellar tumor (Figure 1) that exhibited characteristics of a meningioma. A complete blood count revealed a mild normocytic anemia with hemoglobin of 11.0 mg/dl, and a chemistry panel with ionized calcium was normal. Pre-operative steroids were administered, and the mass was resected with satisfactory post-operative course and resolution of neurologic symptoms. Pathology revealed a metastatic poorly-differentiated carcinoma. Immunohistochemical stains were positive for AE1/AE3, CDX2, CK7, CK20, and CAM5, consistent with a non-neuronal, gastrointestinal origin.
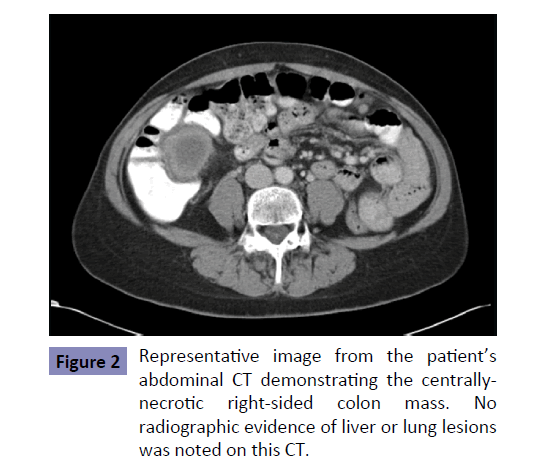
A subsequent CT of her chest, abdomen, and pelvis revealed a 4.6 × 4.2 × 2.7 cm centrally necrotic mass along the medial aspect of the cecum and ascending colon, concerning for a primary colon malignancy. Interestingly, no liver, lung, or bone metastases were observed (Figure 2). An ensuing colonoscopy revealed a lesion in the ascending colon and a biopsy confirmed an intermediategrade adenocarcinoma. She had never undergone screening colonoscopy prior to her diagnosis.
Post-craniotomy, she underwent whole-brain radiation therapy, followed by right-sided hemi-colectomy. Pathologic evaluation revealed invasion into peri-colonic adipose tissue with 7/22 mesenteric lymph nodes positive for adenocarcinoma, consistent with pT3N2bM1 stage IV disease. She underwent 12 cycles of adjuvant modified FOLFOX6 chemotherapy without complications.
Thirteen months later, multiple 1.5 cm hepatic lesions were discovered on a surveillance CT abdomen. Biopsy and mutation analysis revealed KRAS wild type metastatic colorectal cancer. She was re-initiated on FOLFOX6+bevacizumab therapy, but developed a significant reaction during the second oxaliplatin infusion, prompting a switch to FOLFIRI+bevacizumab. Progression of liver metastases after 2 cycles of this regimen was observed, prompting replacement of bevacizumab with cetuximab, and continuation of FOLFIRI. After 7 months of disease response with FOLFIRI+cetuximab, a CT abdomen revealed progression of liver disease. Therapy was switched to regorafenib. At the time of submission, she had completed 2 months of this treatment 30 months after initial diagnosis.
Discussion
Colorectal cancer is the third most common malignancy and the third most common cause of cancer death in the United States [1]. Brain metastasis occurs in approximately 1.5% of all colorectal cancer (CRC) cases, but this percentage is expected to increase as improving treatment options extend overall survival for patients with metastatic colorectal cancer (mCRC) [2-7]. Brain metastases are approximately three times more likely to arise from distal than proximal colon primary sites [8], partially owing to the distal anatomic overlap of portal and systemic circulation. CRC brain metastasis typically occurs in the setting of concurrent extra-cranial metastases, with liver, lung, and bone being the most common sites [2,3,5-7]. Brain metastasis in the absence of other extra-colonic locations is rare, occurring in less than 0.5% CRC cases. With respect to brain metastases, infra-tentorial lesions are observed in less than 20% of brain metastases from mCRC [2-7] (Table 1). Furthermore, the median time from diagnosis of CRC to brain metastasis occurrence has been reported to be between 22 and 36 months [9], unlike our case where brain metastasis represented the initial manifestation of metastatic CRC (mCRC). Thus, this case of mCRC originating from the proximal colon, presenting initially as an isolated, infratentorial, synchronous brain metastasis represents a particularly uncommon presentation of an otherwise common malignancy.
| Onodera et al. [3] |
Nieder et al. [4] |
Jung et al. [5] |
Noura et al. [6] |
Damiens et al. [7] |
|
|---|---|---|---|---|---|
| # Patients | 17 | 35 | 126 | 29 | 48 |
| Mean Age, years | 59 | 63 | 62 | 58 | 63 |
| Male:Female ratio | 3.2 | NR | 1.8 | 3.8 | 1.1 |
| Incidence of BM in CRC, % | 1.6 | NR | 1.6 | 1.3 | NR |
| Primary tumor site, % | |||||
| Ascending+transverse | 0 | NR | 24 | NR | 40 |
| Descending+sigmoid | 29 | NR | 13 | NR | 12 |
| Rectum | 71 | NR | 63 | 59 | 48 |
| BM characteristics, % | |||||
| Single | NR | 49 | 40 | 31 | 63 |
| Multiple | 76 | 51 | 60 | 69 | 37 |
| Supra-tentorial only | NR | NR | 74 | 48 | 54 |
| Infra-tentorial only | 29 | NR | 18 | 6 | 23 |
| Concurrent met site, % | |||||
| Lung | 76 | NR | 72 | 69 | 64 |
| Liver | 47 | NR | 33 | 24 | 50 |
| Lung+Liver | NR | NR | NR | NR | NR |
| Bone | 23 | NR | 21 | 6.8 | NR |
| None | 12 | 34 | 8 | 21 | 10 |
| Median survival after BM, months (range) |
|||||
| Overall median survival | 4.5 (NR) | 5 | 5.4 (3.9-6.9) | 7.4 (NR) | 4 (1-13) |
| Surgery only | 5.2* (NR) | NR | 11.5** (0.2-22.7) | 5.1 (NR) | 3 (NR) |
| WBRT | 4.5 (NR) | 3 (1-8) | 4 (1.4-6.5) | 7.9 (NR) | 4 (NR) |
| SRS | 5.2* (NR) | NR | 9.5 (4.7-14.2) | 7 (NR) | NR |
| Surgery+Radiation | NR | 9* (2-24) | 11.5** (0.2-22.7) | 11.4*** (NR) | 13 (NR) |
| Supportive cares | 3.7 (NR) | NR | 1.5 (1.1-1.9) | 1.6 (NR) | 2 (NR) |
| Chemotherapy after BM | NR | NR | 12.7 (9.5-16.0) | NR | NR NR |
*Patients who received "surgery or radiosurgery" were combined into the same analysis group.
**Patients who received "surgery +/- WBRT" were combined into the
same analysis group.
***Patients received surgery plus either WBRT or SRS.
Table 1: Tumor characteristics from selected studies with BM in mCRC, with references. BM: Brain metastasis; NR: not reported; WBRT: Whole brain radiation therapy; SRS: Stereotactic radiosurgery.
Prognosis is generally poor in patients with brain metastases in mCRC, with a median overall survival in the range of 5 months (range 4.5-7.4 months, Table 1). Neurosurgical resection of brain metastasis followed by whole brain radiotherapy (WBRT) may improve survival by up to approximately 6 months in patients with mCRC, compared to either surgery or radiation alone [3-7,10-13]. Survival outcomes with various approaches to CRC brain metastases are listed in Table 1. Infratentorial metastasis has been shown to be a poor prognostic factor when compared to supratentorial metastasis (5.1 vs 9.1 months). Other factors such as gender and number of metastases do not appear to impact overall survival after neurosurgical resection of brain metastasis [14].
Limited data exists on which systemic agents are optimal in the first-line treatment for CRC brain metastases [4,15-17]. The standard options include oxaliplatin or irinotecan combined with 5-fluoruracil/leucovorin plus a biologic agent targeting the vascular endothelial growth factor (VEGF) or epidermal growth factor recpeptor (EGFR) pathways. For those with RAS wildtype cancers, no data has been reported on the effectiveness of panitumumab or cetuximab in CRC brain metastases. The frequency of KRAS mutations among patients with CRC brain metastases has been reported at 56%, somewhat higher than the frequency of KRAS mutations reported for mCRC in general (40%) [18]. Since patients with KRAS mutations are not candidates for treatment with the EGFR antagonists cetuximab or panitumumab, these therapies may play less of a role in patient with CRC brain metastases [19].
Bevacizumab, a humanized monoclonal antibody with activity against vascular endothelial growth factor A ligand, has been approved as first and second-line therapy in mCRC when combined with cytotoxic chemotherapy. However, the utility of this agent for treating brain metastases remains unclear, as an early case of intra-cerebral hemorrhage after bevacizumab administration led to the exclusion of patients with BM from virtually all subsequent clinical trials [20-23]. Importantly, in a 2008 case report, this complication was not observed in a patient with mCRC and known brain metastasis when bevacizumab was added to salvage 5-FU-based therapy [20]. Ongoing studies with gliomas and breast cancer are providing similar results, which may renew enthusiasm for more rigorous clinical investigation of bevacizumab and other targeted agents in the treatment of brain metastases in mCRC [23-25].
We present an uncommon case of isolated symptomatic infratentorial brain metastasis as the initial presentation of rightsided mCRC in an otherwise healthy 55 year-old female. Despite the usually poor prognosis associated with infra-tentorial brain metastasis in mCRC, she was alive and continuing palliative therapy 30 months after diagnosis. This case further highlights the benefit of combined versus single modality treatment for brain metastases in mCRC. The role of biologic agents in the treatment of brain metastases remains unclear, warranting further study in this patient population.
References
- Siegel RL, Miller KD, Jemal A (2015) Cancer statistics, 2015, CA. A Cancer Journal for Clinicians 65: 5-29.
- Ko FC, Liu JM, Chen WS, Chiang JK, Lin TC, et al. (1999) Risk and patterns of brain metastases in colorectal cancer: 27-year experience. Diseases of the Colon & Rectum 42: 1467-1471.
- Onodera H, Nagayama S, Tachibana T, Imamura I (2005) Brain metastasis from colon cancer. International Journal of Colorectal Disease 20: 57-61.
- Nieder C, Pawinski A, Balteskard L (2009) Colorectal cancer metastatic to the brain: time trends in presentation and outcome. Oncology 76: 369-374.
- Jung M, Bae AJ, Chang JH, Suh CO, Hong SR, et al. (2011) Brain Metastases from colorectal carcinoma: prognostic factors and outcome. Journal of Neurooncology 101: 49-55.
- Noura S, Ohue M, Shingai T, Fujiwara A, Imada S, et al. (2012) Brain metastasis from colorectal cancer: prognostic factors and survival. Journal of Surgical Oncology 106: 144-148.
- Damiens K, Ayoub JPM, Lemieux B, Aubin F, Saliba W, et al. (2012) Clinical features and course of brain metastases in colorectal cancer: an experience from a single institution. Current Oncology 19: 254-258.
- Baek JY, Kang MH, Hong YS, Kim TW, Kim DY, et al. (2011) Characteristics and prognosis of patients with colorectal cancer-associated brain metastases in the era of modern systemic chemotherapy. Journal of Neurooncology 104: 745-753.
- Go PH, Klaassen Z, Meadows M, Chamberlain RS (2011) Gastrointestinal cancer and brain metastasis, a rare and ominous sign. Cancer 117: 3630-3640.
- Vecht CJ, Haaxma-Reiche H, Noordijk EM, Padberg GW, Voormolen JH, et al. (1993) Treatment of single brain metastasis: Radiotherapy alone or combined with neurosurgery?. Annals of Neurology 33: 583-590.
- Aprile G, Zanon E, Tuniz F, Iaiza E, De Pauli F, et al. (2009) Neurosurgical management and postoperative whole-brain radiotherapy for colorectal cancer patients with symptomatic brain metastases. Journal of Cancer Research and Clinical Oncology 135: 451-457.
- Hammoud MA, McCutcheon IE, Elsouki R, Schoppa D, Patt YZ (1996) Colorectal carcinoma and brain metastasis: distribution, treatment, and survival. Annals of Surgical Oncology 3: 453-463.
- Farnell GF, Buckner JC, Cascino TL, O’Connell MJ, Schomberg PJ, et al. (1996) Brain metastases from colorectal carcinoma. The long-term survivors. Cancer 78: 711-716.
- Wronski M, Arbit E (1999) Resection of brain metastases from colorectal carcinoma in 73 patients. Cancer 85: 1677-1685.
- Ciliberto D, Prati U, Roveda L, Barbieri V, Staropoli N, et al. (2012) Role of systemic chemotherapy in the management of resected or resectable colorectal liver metastases: a systematic review and meta-analysis of randomized controlled trials. Oncology Reports 27: 1849-1856.
- Kuebler JP, Wieand HS, O'Connell MJ, Smith RE, Colangelo LH, et al. (2007) Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. Journal of Clinical Oncology 25: 2198-2204.
- Cassidy J, Clarke S, Diaz Rubio E, Scheithauer W, Figer A, et al. (2008) Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. Journal of Clinical Oncology 26: 2006-2012.
- Tie J, Lipton L, Desai J, Gibbs P, Jorissen RN, et al. (2011) KRAS mutation is associated with lung metastasis in patients with curatively resected colorectal cancer. Clinical Cancer Research 17: 1122-1130.
- Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, et al. (2008) Wild-Type KRAS is Required for Panitumumab Efficacy in Patients With Metastatic Colorectal Cancer. Journal of Clinical Oncology 26: 1626-1634.
- Caffo M, Baresi V, Caruso G, Cutugno M, La Fata G, et al. (2013) Innovative therapeutic strategies in the treatment of brain metastases. International Journal of Molecular Sciences 14: 2135-2174.
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, et al. (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. New England Journal of Medicine 350: 2335-2342.
- Hurwitz HI, Fehrenbacher L, Hainsworth JD, Heim W, Berlin J, et al. (2005) Bevacizumab in combination with fluorouracil and leucovorin: an active regimen for first-line metastatic colorectal cancer. Journal of Clinical Oncology 23: 3502-3508.
- Bhaskara A, Eng C (2008) Bevacizumab in the treatment of a patient with metastatic colorectal carcinoma with brain metastases. Clinical Colorectal Cancer 7: 65-68.
- Besse B, Lasserre SF, Compton P, Huang J, Augustus S, et al. (2010) Bevacizumab safety in patients with central nervous system metastases. Clinical Cancer Research 16: 269-278.
- Lu YS, Chen WW, Lin CH, Yeh DC, Tseng LM, et al. (2015) Bevacizumab preconditioning followed by Etoposide and Cisplatin is highly effective in treating brain metastases of breast cancer progressing from whole-brain radiotherapy. Clinical Cancer Research 21: 1851-1858.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences