Multicenter Randomized Controlled Clinical Trial Comparing Conventional and Lateral Invagination Techniques for Double Stapling Colorectal Anastomosis: The ILAC Trial
Carolina González-Abós, Paula Domínguez-Garijo, Yoelimar Guzmán, Ana Otero-Piñeiro, Raquel Bravo, Antonio M. Lacy and F. Borja de Lacy
Department of Gastrointestinal Surgery, Hospital Clinic of Barcelona, Barcelona, Spain
Received Date: 2022-08-24 | Published Date: 2022-09-28DOI10.4172/2471-9943.8.1.001
Carolina González-Abós, Paula Domínguez-Garijo, Yoelimar Guzmán, Ana Otero-Piñeiro, Raquel Bravo, Antonio M. Lacy and F. Borja de Lacy*
Department of Gastrointestinal Surgery, Hospital Clinic of Barcelona, Barcelona, Spain
- *Corresponding Author:
- F. Borja de Lacy
Department of Gastrointestinal Surgery,
Hospital Clinic of Barcelona,
Barcelona,
Spain
E-mail: delacy@clinic.cat
Received date: 24-Aug-2022, Manuscript No. IPJCC-22-14431; Editor assigned: 29-Aug- 2022, PreQC No. IPJCC-22-14431 (PQ); Reviewed: 12-Sep-2022, QC No IPJCC-22-14431; Revised: 20-Sep-2022, Manuscript No. IPJCC-22-14431 (R); Published: 28-Sep-2022, DOI: 10.4172/2471-9943.8.1.001
Citation: Caroflfina Gonzáflez-Abós, Paufla Domínguez-Garijo, Yoelimar Guzmán, Ana Otero-Piñeiro, Raquel Bravo, et al. (2022) Multicenter Randomized Controlled Clinical Trial Comparing Conventional and Lateral Invagination Techniques for Double Stapling Colorectal Anastomosis: The ILAC Trial. Colorectal Cancer Vol. 8 S1: 001.
Abstract
Background: Anastomotic leakage is a severe complication in colorectal surgery, occurring in up to 20% of all procedures. The double stapling technique is among the most commonly preferred methods, although the extremes of the linear suture line (called “dog ears”) and the number of staple lines may affect the leak rate. Observational retrospective studies point out the lateral invagination of the anastomotic edges as a potential solution.
Methods: The ILAC trial is a multicenter, randomized, single-blind superiority study designed with the intent of evaluating the effectiveness and safety of the lateral invagination technique for the double-stapled colorectal anastomosis. Two groups (conventional, group 1; and lateral invagination, group 2) will be compared. All adult patients admitted to the participating centers with an indication for elective resection of the left colon, sigmoid or upper rectum will be included. With an alpha risk of 0.05 and a beta risk of 0.20 with bilateral contrast, we aim to include 393 patients per group. The analyses will be performed by the intention to treat principle. The primary outcome is the rate of anastomotic leakage. Secondary outcomes include duration of surgery, perioperative morbidity using the Clavien- Dindo classification, hospital stay, hospital readmissions and reinterventions.
Discussion: Aiming to reduce controllable factors related to suture dehiscence rates, different intraoperative technique modifications have been developed. The lateral invagination of the “dog-ears” in double-stapled colorectal anastomosis appears to be a potentially beneficial modification in reducing anastomosis failure. However, no clinical trials have been developed to prove this. In response to these needs, the present study aims to evaluate the role of the lateral invagination technique in a randomized and controlled trial.
Keywords
Colorectal cancer; Anastomotic leakage; Lateral invagination technique; Double-stapled colorectal anastomosis
Introduction
Background
Anastomotic leakage is the most feared complication in colorectal surgery, occurring in 6%-13% in patients with pelvic anastomoses [1-5]. This complication significantly increases morbidity, mortality, costs, and generates a greater impact on quality of life [6]. In addition, several studies point to an increased risk of loco regional recurrence [7].
There are different risk factors for anastomotic leak: Preoperative, such as malnutrition or obesity; intraoperative, such as hypoperfusion or the anastomotic technique; and postoperative, such as some types of medication [8]. In colorectal anastomoses, there is some concern about the safety of the double stapling technique, since the extremes of the linear suture line (called “dog ears”) and the number of staple lines may affect the leak rate [9-12]. With the aim of reducing anastomotic leak rates, different intraoperative techniques have been developed, such as suture reinforcement, the use of indocyanine green or the application of anastomotic sealants [13-15]. However, a definitive solution has not been found. Recently, benefits have been suggested with the lateral invagination of the double-stapled colorectal anastomosis, with the aim of avoiding the “dog ears” [16-18]. Several case series and retrospective comparative studies have shown a significant decrease in anastomotic dehiscence with this technique, with leak rates of 0.0%-0.8% [16-18].
Therefore, the present study aims to evaluate the effectiveness and safety of the lateral invagination technique of the double-stapled colorectal anastomosis in a randomized and controlled trial. In this sense, our primary hypothesis is that the lateral invagination reduces the incidence of anastomotic leak compared to the conventional technique.
Materials and Methods
Study protocol has been developed according to SPIRIT recommendations (Table 1).
| STUDY PERIOD | |||||||
|---|---|---|---|---|---|---|---|
| Enrolment | Allocation | Post-allocation | Close-out | ||||
| Deciding indication for surgery | 0 | Day before surgery | Surgery | In-hospital | 30day | 30 day or after stoma closure | |
| TIMEPOINT | |||||||
| ENROLMENT | |||||||
| Eligibility screen | X | ||||||
| Informed consent | X | ||||||
| Randomization | X | ||||||
| Allocation | X | ||||||
| INTERVENTIONS | |||||||
| Preoperative management according to each center | X | ||||||
| Conventional technique | X | ||||||
| Invagination technique | X | X | |||||
| ASSESSMENTS | |||||||
| Inclusion criteria | X | ||||||
| Exclusion criteria | X | ||||||
| Preoperative variables | X | X | |||||
| Intraoperative variables | X | X | |||||
| Primary endpoint | X | X | |||||
| Postoperative variables | X | X | X | X | |||
Table 1: SPIRIT template for ILAC Trial Protocol.
Study design
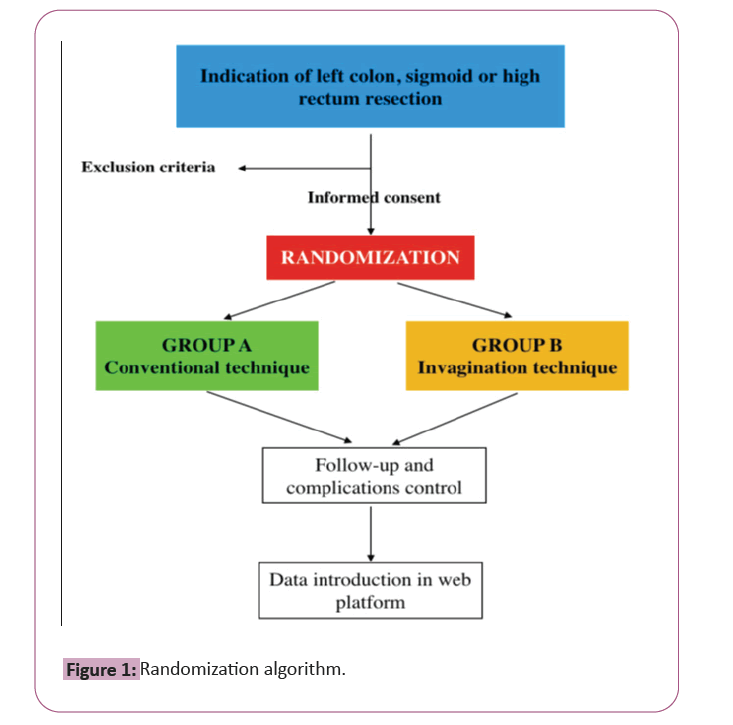
Multicenter, randomized, single-blind superiority study designed with the intent of evaluating the effectiveness and safety of the lateral invagination technique for the double-stapled colorectal anastomosis. Two groups (conventional, group 1; and lateral invagination, group 2) will be compared. During the study, the clinical data will be analyzed in a centralized and anonymized way to ensure its uniformity.
Study population
All adult patients admitted to the participating centers (Table 2) with an indication for elective resection of the left colon, sigmoid or upper rectum (in the case of oncological pathology with the primary tumor with the lower border above the level of the “sigmoid take off” according to the international consensus definition of the rectum)[19].
| Center/Hospital | Local Leader | Country |
|---|---|---|
| Hospital Clinic de Barcelona (Leader) | Carolina González-Abós | Barcelona (Spain) |
| Complejo Hospitalario Universitario de Vigo | LucÃa Garrido | Vigo (Spain) |
| Hospital ClÃnico Universitario de Santiago | Jesús Paredes | Santiago (Spain) |
| Hospital Universitario del Sureste | Manuel Losada | Madrid (Spain) |
| Hospital Virgen de la Arrixaca | Elena Gil | Murcia (Spain) |
| Hospital Germans Trias i Pujol | Marta Cuadrado | Barcelona (Spain) |
| National Cancer Center Hospital East | Daichi Kitaguchi | Japan |
Table 2: Participating centers active in patient inclusion.
Inclusion criteria:
• Age>18 years
• Indication for elective resection of the left colon, sigmoid or upper rectum
• Surgery by minimally invasive or open approach
• Double-stapled end-to-end colorectal anastomosis
• Signed informed consent for inclusion in the study
Exclusion criteria:
• Patients <18 years
• Pregnancy or breastfeeding
• ASA> III
• Absolute contraindication for anesthesia
• Patients receiving more than 1 gastrointestinal anastomosis during the same procedure
• Planned multi-organ resection during the same procedure
• Urgent / emergent surgery
• Patients with simultaneous application of debulking and/or HIPEC
• Crohn’s disease or active ulcerative colitis
Randomization procedure
Randomization will be carried out centralized to avoid selection bias. The randomization is computer-based without patient stratification. Each participant center will be able to communicate electronically the existence of a patient likely to enter the study. Once the patient meets the inclusion requirements, he/she must sign the informed consent for the study. With the signed consent, the participating center should communicate electronically the inclusion of the patient. Immediately the case number and the randomization group will be assigned. Patients must also sign the consent for the surgery.
Randomization groups
Randomization will assign patients who meet the inclusion criteria. The randomization will be a 1:1 ratio between the two groups following the algorithm showed in Figure 1.
Intervention: Surgical procedure
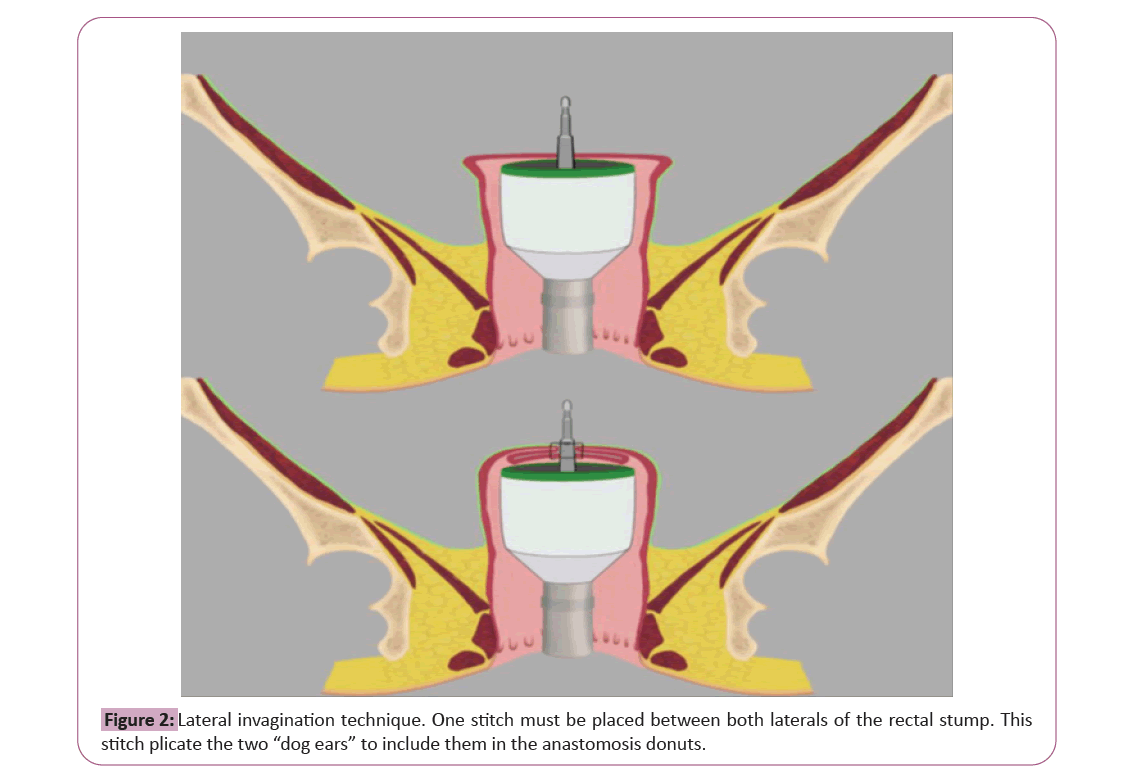
Resection of the left colon, sigmoid or upper rectum according to convenience through open surgery or minimally invasive approach following the standards of each center. The section of the distal end of the surgical specimen will be made at the level of the rectum with a linear stapler. The colorectal anastomosis will be performed with a mechanical circular endostapler in all cases, with insertion of the endostapler through the rectal stump and placement of the anvil at the proximal colonic stump. The use of double-edged circular anastomosis is widely accepted by the scientific and surgical community, while the modification proposed in this study has reported better results in all previous publications and in no case it seems to increase the rate of suture dehiscence in patient groups analyzed. The anastomotic technique will depend on the group after randomization; (group 1) the circular endostapler will be fired in a conventional way, that is, without having invaginated the two corners of the staple line; (group 2) double-staple colorectal anastomosis will be performed following the technique [15]. prior to firing, a suture will be placed at the rectal stump that includes both “dog ears”. After the punch comes out of the endostapler, the stitch will be tied, which will invaginate the two corners of the staple line on the same punch. Subsequently, the endostapler will be closed and fired, including the “dog ears” in the anastomotic rims as shown in Figure 2 [16]. This technical modification is an easy task for a colorectal surgeon and prior virtual training will be performed. The rest of the surgery will be performed according to the standards of each center. Completeness of donoughts will be assessed and recorded.
Postoperative follow-up
A postoperative follow-up with clinical control will be carried out during hospital admission. Control of vital signs will be carried out periodically, removal of the foley catheter when an adequate diuresis is verified, and initiation of oral diet in the presence of physiological peristalsis. Given the clinical suspicion of anastomotic dehiscence (which includes signs such as increase of abdominal pain, peritonism, elevation of acute phase reactants, fever or intestinal material leakage through the adominal drainage) the attitude will depend on the standards of each center. Usually, a CT scan with endorectal contrast or a direct surgical intervention will be performed. After discharge, there will be no additional or different tests to the conventional follow-up. A 30-day follow-up will be carried out in patients with primary anastomosis and no diverting stoma.
Study parameters
Patients’ characteristics: Collected patient characteristics will gender, age, height, weight, ASA classification, WHO performance status, relevant medical history, height of colorectal resection, surgical approach (e.g., minimally invasive versus open), extent of resection, level of vascular ligation and splenic flexure mobilization, type of anastomosis and distance from the anal verge, primary and secondary defunctioning stoma.
Anastomotic leakage and complications: Anastomotic leakage, defined as a defect of the intestinal wall integrity at the colorectal anastomotic site leading to a communication between the intra- and extraluminal compartments. A pelvic abscess close to the anastomosis is also considered as anastomotic leakage [20,21].
Grades:
A. Anastomotic leakage requiring no active therapeutic intervention
B. Anastomotic leakage requiring active therapeutic intervention but manageable without relaparotomy
C. Anastomotic leakage requiring re-laparotomy
Although the primary stoma protection rate is estimated to be low, anastomotic dehiscence will also be considered in those cases in which the clinical or radiological manifestations only become evident after transit reconstruction (silent leak). Other complications such as surgical site infection, bleeding, sepsis and the rest included in the Clavien-Dindo classification will also be registered.
Outcome parameters: The primary outcome is the rate of anastomotic leakage in each group. Secondary outcomes include duration of surgery, perioperative morbidity and mortality, hospital stay, hospital readmissions and reinterventions.
Sample size calculation: In the literature, the pelvic anastomotic leak rate ranges between 6.3% and 13.7% [1-5]. According to the study a leak rate decrease of more than 4% is expected with the lateral invagination technique [16]. Therefore, in this study we established the expected difference between the groups of 4%.
With an alpha risk of 0.05 and a beta risk of 0.20 with bilateral contrast, a sample calculation of 393 patients per group was obtained.
Schedule: Taking into account the sample size (n=786) and the multicenter design of the study, the inclusion period is estimated to be 1.5 years. Likewise, with the primary objective of anastomotic leak in the first 30 postoperative days, it is estimated to obtain definitive results within 2 years (Figure 2). It is planned to perform an intermediate analysis when reaching 50% of inclusions.
The investigator will submit a summary of the trial progress to the accredited medical ethics committee once a year. Information on the date of inclusion of the first subject, the number of subjects included and the number of subjects who completed the trial, serious adverse events/serious adverse reactions/other problems and amendments will be provided.
All medical data will be collected by the main coordinating center. Data collection will be facilitated through online case registration forms for the perioperative period. For patient privacy, hospital patient identification numbers will not be disclosed to the coordinating center. All patient data is coded and identified by a randomization number. This randomization number does not include the patient's initials or date of birth. The local investigator will have a decoding list with randomization numbers and hospital patient identification numbers of their patients on file at the investigator site. At each trial operation, the participating surgeon or surgeons are noted on the case record form. All patients considered for surgical resection of the left colon, sigmoid or upper rectum electively should be registered, including those who declined randomisation and those who did not meet the inclusion criteria. Brief details of the reasons why patients are not randomized or excluded should be given. The number of patients operated on in each center will be recorded. All collaborating centers have signed the required contract for patient’s inclusion and data protection.
Results
Statistical data management
Data collection: Only the data gathered routinely will be collected in the study, and are indicated in Annex 1. The data will be entered by a member responsible for each center in the assigned web platform with the identification code sent at the time of randomization.
Patients will not undergo any additional investigation for the purposes of this study. The clinical follow-up will be limited to the review of health records. There will be no additional contact with the patient (by phone or in person) beyond normal clinical practice at each center. No identifiable data will be collected in the database, and the patient's clinical team will only upload anonymized data.
Statistical analysis: Main analyses will be performed by intention to treat principle. Quantitative variables will be described as mean and standard deviation or as median and interquartile range and inferential analyses will be performed using t-test for independent groups or Mann-Whitney U test respectively. Qualitative variables will be expressed as relative and absolute frequencies and analyzed using Fisher’s exact test. Ordinal variables will be analyzed by Mann-Whitney U test. A prespecified analysis by subgroups will be performed according to: Volume of the center of origin, type of surgery, presence or absence of a stoma, and surgical approach. No evaluable patients or missing data for main outcome will be imputed to failure. For the rest of variables no formal imputations will be performed and the analyses will be based on the Available Data Only (ADO) approach. There will be a blinded assessment, Data Blind Review (DBR), of the results after the last patient in the trial has completed follow-up (Table 3).
| Months | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 4 | 6 | 8 | 10 | 12 | 16 | 18 | 22 | 24 | |
| Multi-institucional agreement |  |
 |
|||||||||
| On-line platform development |  |
 |
|||||||||
| Patient inclusion period |  |
 |
 |
 |
 |
 |
 |
||||
| Surgical procedures |  |
 |
 |
 |
 |
 |
 |
||||
| Intermediate analysis |  |
||||||||||
| Data collection |  |
 |
 |
 |
 |
 |
 |
||||
| Statistic analysis |  |
 |
|||||||||
| Project evaluation |  |
 |
|||||||||
| Manuscript edition |  |
||||||||||
| Barcelona (Spain) |  |
 |
|||||||||
Table 3: Estimated scheduling for ILAC Trial. Note:  Months of results obtained.
Months of results obtained.
For the main outcome (leak rate between both techniques), statistical significance will only be considered at two-sided Type I Error of 5%. An interim analysis will be performed when 50% of evaluable patients are available. To maintain a global Type I Error of 5%, an adjustment is proposed by the O'Brien method-Fleming, so that the first cut-off will be considered statistically significant if the value of p is less than or equal to 0.005. In case of not obtaining a statistically significant result, the recruitment will continue. The final analysis will be carried out by comparing the value of p with a limit of 0.049. For the rest of statistical analyses p-values will be considerated as nominal for descriptive purposes only. The data will be analyzed using the IBM SPSS Statistics program, version 25 or higher (IBM Corp., Armonk, NY, USA).
Ethical aspects of the study
The study is designed in such a way that at no time is a variation in the routine management of the patient. The screening and treatment of complications will be carried out as is done in current clinical practice. Moreover, the use of double-stapled circular anastomosis is widely accepted by the scientific community and the modification proposed in this study has reported better results in all previous publications and in no case does it seem to increase the rate of suture dehiscence in patients’ groups analyzed [16-18].
The research team will carry out the study in accordance with the protocol, the principles established in the current revised version of the Declaration of Helsinki (Seoul, 2008) and in accordance with the standards of Good Clinical Practice, as described in the Harmonized Tripartite Standards of the ICH for Good Clinical Practice (1996) and the guidelines for Good Epidemiological Practice (http://www.ieaweb.org/GEP07.htm). The study will act in compliance, in accordance with the Biomedical Research Law 14/2007. This study was approved by the Clinical Research Ethics Committee (CEIC) of the Hospital Clinic de Barcelona, an independent committee that ensures respect for the rights, well-being and safety of patients in biomedical research projects. Likewise, it must be approved by the Ethics Committee of all participating Hospitals.
Discussion
Anastomotic leak in colorectal surgery remains an unsolved problem. This dreaded complication, present in a considerable percentage of patients, entails an increase in morbidity, mortality and costs over the standard procedure, as well as a greater impact on quality of life [1-7]. Previous research has mainly focused on incidence and establishment of risk factors, being the majority barely modifiable [1,3]. Aiming to reduce controllable factors related to suture dehiscence rates, different intraoperative technique modifications have been developed [13,14,16,18]. Nevertheless, due to the observational and retrospective nature of these studies, none of them have managed to rise as a routine procedure for prevention of anastomotic leak.
One of the elements in the spotlight is the number of concurrent staple lines, associated with the “dog ears” that are created at the extremes of the linear suture line, as it has been directly related with the risk of dehiscence, especially in case of colorectal end-to-end anastomoses [11-12]. The lateral invagination of these “dog-ears” appears to be a potentially beneficial modification in reducing anastomosis failure but no clinical trials have been developed to prove it [16-18]. In response to these needs, the present study aims to evaluate the effectiveness and safety of the lateral invagination technique of double-staple colorectal anastomosis in a randomized and controlled trial.
The main strengths of this study are the prospective and randomized nature and the large number of patients we aim to include. Although different observational studies have already shown benefit of the modification technique we aim to evaluate, we are not aware that there is any randomized study, which is considered gold-standard research design for answering questions about treatment effectiveness [16-18]. Moreover, due to the allocation process design and blinded analysis of the data, selection, attrition and detection bias will be controlled. On the other hand, the inclusion of a high number of patients will be possible by the multicentric collaborative nature of this study, which at the same time may provide generalizability of results to other populations. Perhaps the most important limitation is that this technical modification represents a change from the usual surgical intervention in the majority of the participating centers. Like any surgical procedure, it is expected that surgeons will undergo a learning curve. Nevertheless, considering the experience of the participating surgeons and the relative simplicity of the modification proposed, it is estimated to be a short process.
An important aspect of the present study is the high impact that may imply to prove that the lateral invagination technique, which is relatively simple and quick to perform, is associated with lower rates of anastomotic leak. Demonstrating that this technical variation provides clinical benefits could even make it as the "gold standard" for double-stapled colorectal anastomoses.
What this study adds to the literature
Anastomotic leakage is an important complication related to colorectal surgery that entails an increase in morbidity, mortality and costs over the standard procedure, as well as a greater impact on quality of life. This study aims to establish whether the double staple colorectal anastomosis lateral invagination technique, which is relatively simple and quick to perform, is associated with lower rates of anastomotic dehiscence.
Conclusion
Anastomotic leakage is an important complication related to colorectal surgery that entails an increase in morbidity, mortality and costs over the standard procedure, as well as a greater impact on quality of life. This study aims to establish whether the double staple colorectal anastomosis lateral invagination technique, which is relatively simple and quick to perform, is associated with lower rates of anastomotic dehiscence. The study is designed in such a way that at no time is a variation in the routine management of the patient. The screening and treatment of complications will be carried out as is done in current clinical practice.
Declarations
Ethics
The study protocol and relevant documents have been approved by the medical Ethical Committee of the Hospital Clinic of Barcelona; Study protocol VERSION 7. 07.11.2020 and Patients informed consent VERSION 2. 06.10.20. Attached documents are provided.
Data confidentiality
The study data will be treated in accordance with Organic Law 3/2018, of December 5, on the Protection of Personal Data and guarantee of digital rights and by extension, in accordance with Regulation (EU) 2022/679 of the European Parliament and of the Council of April 27, 2022 on Data Protection (RGPD) and used exclusively for the development and successful completion of the project.
Information sheet and informed consent form
The patient will sign the study informed consent (annex 2) before participating. In the clinical history or medical records of each patient, the process of obtaining informed consent will be documented and it will be indicated that it has been obtained in writing before their participation in the study. Patients may refuse the offer and refuse consent without giving any explanation and without affecting any proposed treatment.
Contributors
CG-A, PD, YG, FBL and AO-P were responsible for the study design. FBL and AML approved the definitive work protocol.
CG-A, PD and YG drafted the work while FBL, AO-P, RB, RA and AML revised it critically for important intellectual content.
All contributors approved the final version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
The ILAC Trial received a grant from the Spanish Surgical Society (Asociación Española de Cirujanos). There are no companies involved in the conduct or analysis of the study or interpretation of the data.
Data availability: All relevant data related to study protocol will be attached to the submitted manuscript and will be public in Clinicaltrials.gov (NCT 04553250).
Competing interests
Dr. Carolina González-Abós, Paula Domínguez-Garijo, Yoelimar Guzmán, Ana Otero-Piñeiro, Raquel Bravo, F. Borja de Lacy have no conflicts of interest or financial ties to disclose. Dr. Antonio M. Lacy reports personal fees from Medtronic, personal fees from Olympus, personal fees from Applied Medical, and personal fees from Conmed, outside the submitted work.
Protocol Version
Version 7. 07th November 2020
Trial registration
Clinicaltrials.gov (NCT 04553250).
References
- Kang CY, Halabi WJ, Chaudhry OO, Pigazzi A, Carmichael JC, et al. (2013) Risk factors for anastomotic leakage after anterior resection for rectal cancer. JAMA Surg 148:65-71.
[Crossref] [Google Schoar] [Pubmed]
- Dulk MD, Marijnen CA, Collette L, Putter H,Pahlman L, et al. (2009) Multicentre analysis of oncological and survival outcomes following anastomotic leakage after rectal cancer surgery. Br J Surg 96:1066-1075.
[Crossref] [Google Schoar] [Pubmed]
- Peeters KC, Tollenaar RA, Marijnen CA,Wiggers T, Rutten HJ, et al. (2005) Risk factors for anastomotic failure after total mesorectal excision of rectal cancer. Br J Surg 92:211-216.
[Crossref] [Google Schoar] [Pubmed]
- Paun BC, Cassie S, MacLean AR, Dixon E, Donald BW, et al. (2010) Postoperative complications following surgery for rectal cancer. Ann Surg 251:807-818.
[Crossref] [Google Schoar] [Pubmed]
- Sciuto A, Merola G, Palma GDD, Sodo M, Pirozzi F, et al. (2018) Predictive factors for anastomotic leakage after laparoscopic colorectal surgery. World J Gastroenterol 24:2247-2260
[Crossref] [Google Schoar] [Pubmed]
- Kingham TP, Pachter HL. (2009) Colonic anastomotic leak: Risk factors, diagnosis, and treatment. J Am Coll Surg 208:269-278
[Crossref] [Google Schoar] [Pubmed]
- Mirnezami A, Mirnezami R, Chandrakumaran K, Sasapu K, Sagar P, et al. (2011). Increased local recurrence and reduced survival from colorectal cancer following anastomotic leak: Systematic review and meta-analysis. Ann Surg 253:890–899.
[Crossref] [Google Schoar] [Pubmed]
- Gorissen KJ, Benning D, Berghmans T, Snoeijs MG, Sosef MN, et al. (2012) Risk of anastomotic leakage with non-steroidal anti-inflammatory drugs in colorectal surgery. BJS 99:721-727
[Crossref] [Google Schoar] [Pubmed]
- Ito M, Sugito M, Kobayashi A, Saito N, Tsunoda Y, et al. (2008) Relationship between multiple numbers of stapler firings during rectal division and anastomotic leakage after laparoscopic rectal resection. Int J Colorectal Dis 23:703-707.
[Crossref] [Google Schoar] [Pubmed]
- Kim JS, Cho SY, Min BS, Kim NK. (2009) Risk factors for anastomotic leakage after laparoscopic intracorporeal colorectal anastomosis with a double stapling technique. J Am Coll Surg 209: 694–701
[Crossref] [Google Schoar] [Pubmed]
- Park JS, Choi GS, Kim SH, Kim HR, Lee KY, et al. (2013) Multicenter analysis of risk factors for anastomotic leakage after laparoscopic rectal cancer excision: The Korean laparoscopic colorectal surgery study group. Ann Surg 257:665-671.
[Crossref] [Google Schoar] [Pubmed]
- Kawada K, Hasegawa S, Hida K, Hirai K, Sakai Y, et al. (2014) Risk factors for anastomotic leakage after laparoscopic low anterior resection with DST anastomosis. Surg Endosc 28:2988-2995.
[Crossref] [Google Schoar] [Pubmed]
- Boni L, David G, Dionigi G, Rausei S, Cassinotti E, et al.(2016) Indocyanine green-enhanced fluorescence to assess bowel perfusion during laparoscopic colorectal resection. Surg Endosc 30:2736-2742.
[Crossref] [Google Schoar] [Pubmed]
- James DRC, Ris F, Yeung TM, Kraus R, Buchs NC, et al. (2015) Fluorescence angiography in laparoscopic low rectal and anorectal anastomoses with pinpoint perfusion imaging a critical appraisal with specific focus on leak risk reduction. Colorectal Dis 3:16-21.
- Stergios K, Kontzoglou K, Pergialiotis V, Korou LM, Lalude O, et al. (2017) The potential effect of biological sealants on colorectal anastomosis healing in experimental research involving severe diabetes. Ann R Coll Surg Engl 99:189-192.
[Crossref] [Google Schoar] [Pubmed]
- Lee S, Lee SH, Ahn B (2017) The relationship between the number of intersections of staple lines and anastomotic leakage after the use of a double stapling technique in laparoscopic colorectal surgery. Surg Laparosc Endosc Percutaneous Tech 27(4):273-281.
[Crossref] [Google Schoar] [Pubmed]
- Zhang L, Xie Z, Zang W, Lin H, Lv H (2017) Laparoscopic low anterior resection combined with ‘dog-ear’ invagination anastomosis for mid- and distal rectal cancer. Tech Coloproctol 22:65-68.
[Crossref] [Google Schoar] [Pubmed]
- Chen ZF, Liu X, Jiang WZ, Guan GX (2016) Laparoscopic double-stapled colorectal anastomosis without ‘dog-ears’. Tech Coloproctol 20: 243-247.
[Crossref] [Google Schoar] [Pubmed]
- D'Souza N, Neree Tot Babberich MPMD, Hoore AD, Tiret E, Xynos E, et al. (2019) Definition of the rectum: An international, expert-based delphi consensus. Ann Surg 270:955-959
[Crossref] [Google Schoar] [Pubmed]
- Bannura GC, Cumsille MAG, Barrera AE, Contreras JP, Melo CL, et al. (2006) Factores asociados a la dehiscencia clínica de una anastomosis intestinal grapada: Análisis multivariado de 610 pacientes consecutivos. Rev Chilena de Cirugía 58:341-346
- Rahbari NN, Weitz J, Hohenberger W, Heald RJ, Moran B, et al. (2010) Definition and grading of anastomotic leakage following anterior resection of the rectum: A proposal by the International Study Group of Rectal Cancer. Surgery 147:339-351.
[Crossref] [Google Schoar] [Pubmed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences