Screening for Colorectal Malignancy
Tajamul Hassan, Fazl Qadir Parray, Nisar Ahmad Chowdri and Khursheed Alam Wani
Tajamul Hassan, Fazl Qadir Parray*, Nisar Ahmad Chowdri and Khursheed Alam Wani
Government Housing Colony, Rawal Pora, Sanat Nagar, Srinagar-190 005, Jammu and Kashmir, India
- *Corresponding Author:
- Fazl Qadir Parray
C/O Baitul Qadir, H No 44, Govt Housing Colony, Rawal Pora, Sanat Nagar, Srinagar-190 005, Jammu and Kashmir, India.
E-mail: parray@rediffmail.com
Received date: January 28, 2016; Accepted date: February 22, 2016; Published date: February 29, 2016
Citation: Hassan T, Parray FQ, Chowdri NA. Screening for Colorectal Malignancy. Colorec Cancer 2016, 2:2. doi: 10.21767/2471-9943.100016
Abstract
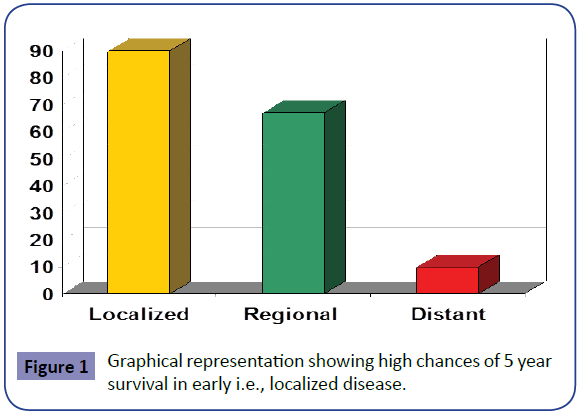
Colorectal cancer is the third most common cancer and the third leading cause of cancer death in men and women in the United States. Despite being so common, colorectal malignancies pose a significant early diagnostic problems due to wide variety of reasons which include lack of awareness of risk factors, avoidance of doctor check-ups, fear of getting tested, perception as “man’s disease”, no symptoms/no problem and lack of proper set-up/colonoscopy especially in remote areas. Numerous international CRC screening programs have been initiated as evidence grows for an impact of CRC screening on mortality. Death from colorectal cancer is preventable. Effective, safe, and relatively inexpensive methods for screening for the disease have been available. The time taken by a sessile polyp to get converted into pedunculated and frank adenocarcinoma is considered as golden period of time for early detection of colorectal malignancies. Even after development of malignancy in the initial stages when the disease is localized the chances of five year survival go as high as 80% and when it has distant metastasis it goes below 10%.
Nearly 90% of colon cancer patients are over the age of 50. Other risk factors include: family or personal history of colon cancer or polyps, chronic inflammatory bowel disease, hereditary colorectal syndromes, use of cigarettes and other tobacco products, high-fat/low fiber diet and physical inactivity.
Screening for colorectal cancer clearly reduces colorectal cancer mortality, yet many eligible adults remain unscreened. Various screening techniques used for detection of colorectal malignancies Fecal occult blood testing (FOBT), Barium enema, Flexible sigmoidoscopy, Colonoscopy, Virtual Colonoscopy, Carcinoembryonic antigen and other tumour markers, Fecal DNA and Genetic testing. The recommendation that all men and women aged 50 years or older undergo screening for colorectal cancer is supported by a large body of direct and indirect evidence.
Colorectal cancer screening has been recommended by the U.S. Preventive Services Task Force (USPSTF) and many other organizations for more than 10 years. On the basis of evidence from multiple randomized, controlled trials (RCTs), a screening program with repeated annual or biennial guaiac fecal occult blood tests (FOBTs) and endoscopic follow-up of positive test results reduces colorectal cancer mortality; according to a recent update, colorectal cancer mortality was reduced 16% (CI, 10% to 22%) after 12 to 18 years. Extrapolating from trial evidence, clinical studies of test accuracy, and other supporting evidence, the USPSTF recognized flexible sigmoidoscopy (with or without FOBTs), colonoscopy, and double-contrast barium enema as other colorectal cancer screening options in 2002. However, because colorectal cancer screening tests have potential harms, limited accessibility, or imperfect acceptability to patients, and no tests could be identified as superior in cost-effectiveness analysis, the USPSTF also recommended that choice among recommended methods for colorectal cancer screening to be individualized to patients or practice settings.
Keywords
Colorectal; Screening; Colonoscopy
Introduction
Colorectal cancer is the third most common cancer and the third leading cause of cancer death in men and women in the United States. The lifetime risk of dying from CRC in the USA is 2.5%. [1]. Globally, CRC is the third most common cancer in men and the second most common in women, with mortality paralleling incidence. 1.2 million new CRC cases and 608,700 related deaths were estimated to have occurred worldwide in 2008 [2]. Despite being so common, colorectal malignancies pose a significant early diagnostic problems due to wide variety of reasons which include: lack of awareness about risk factors, avoidance of doctor check-ups, fear of getting tested, perception as “man’s disease”, no symptoms/no problem and lack of proper set-up/colonoscopy especially in remote areas. Numerous international CRC screening programs have been initiated as evidence grows for an impact of CRC screening on mortality [3].
Incidence and mortality rates
The lifetime probability of a colorectal cancer diagnosis is 4.7% in women and 5.0% in men. Incidence and mortality rates are 30% to 40% higher in men than in women overall. Nearly 90% of colon cancer patients are over the age of 50.Other risk factors include: family or personal history of colon cancer or polyps, chronic inflammatory bowel disease, hereditary colorectal syndromes, use of cigarettes and other tobacco products, high-fat/low fiber diet and physical inactivity.
Principles of screening
Cancer prevention may be categorized as primary or secondary. Primary prevention refers to identifying genetic, biological, and environmental factors that are etiologic or pathogenetic and subsequently altering their effects on tumor development. Although several areas of study have been identified that can lead to primary prevention of large bowel cancer, available data do not yet provide a firm basis for the practical application of primary preventive measures. The goal of secondary prevention is to identify existing preneoplastic and early neoplastic lesions and to treat them thoroughly and expeditiously. The assumption is that early detection improves prognosis.
Benefits of screening
Colon Polyp to Cancer takes about 10-15 years. The time taken by a sessile polyp to get converted into pedunculated and frank adenocarcinoma is considered as golden period of time for early detection of colorectal malignancies. Even after devolpment of malignancy in the initial stages when the disease is localized the chances of five year survival go as high as 80% and when it has distant metastasis it goes below10% as shown graphically in Figure 1.
Screening Techniques: (Table 1)
| Test | Pros | Cons |
|---|---|---|
| Flexible sigmoidoscopy | Fairly quick and safe Usually doesn't require full bowel preparation Sedation usually not used Does not require a specialist Done every 5 years |
Views only about a third of the colon Can miss small polyps Can't remove all polyps May be some discomfort Very small risk of bleeding, infection, or bowel tear Colonoscopy will be needed if abnormal |
| Colonoscopy | Can usually view entire colon Can biopsy and remove polyps Done every 10 years Can diagnose other diseases |
Can miss small polyps Full bowel preparation needed More expensive on a one-time basis than other forms of testing Sedation of some kind is usually needed Will need someone to drive you home You may miss a day of work Small risk of bleeding, bowel tears, or infection |
| Double-contrast barium enema (DCBE) | Can usually view entire colon Relatively safe Done every 5 years No sedation needed |
Can miss small polyps Full bowel preparation needed Some false positive test results Cannot remove polyps during testing Colonoscopy will be needed if abnormal |
| CT colonography (virtual colonoscopy) | Fairly quick and safe Can usually view entire colon Done every 5 years No sedation needed |
Can miss small polyps Full bowel preparation needed Some false positive test results Cannot remove polyps during testing Colonoscopy will be needed if abnormal |
| Guaiac-based fecal occult blood test (gFOBT) | No direct risk to the colon No bowel preparation Sampling done at home Inexpensive |
May miss many polyps and some cancers May produce false-positive test results May have pre-test dietary limitations Should be done every year Colonoscopy will be needed if abnormal |
| Fecal immunochemical test (FIT) | No direct risk to the colon No bowel preparation No pre-test dietary restrictions Sampling done at home Fairly inexpensive |
May miss many polyps and some cancers May produce false-positive test results Should be done every year Colonoscopy will be needed if abnormal |
| Stool DNA test | No direct risk to the colon No bowel preparation No pre-test dietary restrictionsSampling done at home |
May miss many polyps and some cancers May produce false-positive test results Should be done every 3 years Colonoscopy will be needed if abnormal |
Table 1: Screening Techniques.
• Fecal occult blood testing (FOBT).
• Barium enema.
• Flexible sigmoidoscopy.
• Colonoscopy.
• Virtual Colonoscopy.
• Carcinoembryonic antigen and other tumour markers.
• Fecal DNA and Genetic testing.
Fecal occult blood test
For fecal occult blood test three days before and during testing, patients should avoid the following:
For fecal occult blood test three days before and during testing, patients should avoid the following:
• Rare red meat
• Peroxidase-containing vegetables and fruit (e.g., broccoli, turnip, cantaloupe, cauliflower, radish)
• Certain medications (e.g., iron supplements, vitamin C, aspirin and other NSAIDs)
CRCs and adenomas bleed intermittently. Also Hemoccult testing depends on the degree of blood loss. In general, 2 mL of blood in the stool is necessary to produce a positive result. Sampling multiple stool specimens therefore is likely to result in fewer falsenegative evaluations. Sampling one specimen yields a 40% to 50% false-negative rate, which improves progressively as more stools are sampled. Two samples of each of three consecutive (daily) stools should therefore be tested. It should be remembered that in apositive FOBT only 8% will be neoplastic.
Causes of false-positive results:
• Red meat (nonhuman hemoglobin)
• Uncooked fruits and vegetables (vegetable peroxidase: e.g., broccoli, turnip, cantaloupe, cauliflower, radish)
• Any source of GI blood loss (e.g., epistaxis, gingival bleeding, upper GI tract pathology, hemorrhoids)
• Certain medications (e.g., iron supplements, vitamin C, aspirin and other NSAIDs)
• Exogenous peroxidase activity
Causes of false-negative results:
• Storage of slides for a prolonged period
• Degradation of hemoglobin by colonic bacteria
• Ascorbic acid (vitamin C) ingestion
• Improper sampling or developing
• Non-bleeding lesion at the time of stool collection
Air contrast barium enema (ACBE)
If colonoscopy is unavailable, technically difficult, or refused by the patient, ACBE can be done. ACBE traditionally has been performed following sigmoidoscopy. ACBE has been included as an option in a variety of screening guidelines. No studies, however, have directly addressed the effectiveness of barium enema for CRC screening. Several studies have indicated that the sensitivity of ACBE is less than that of colonoscopy, especially for detecting lesions smaller than 1 cm. A population-based study [4] suggested that if a cancer is present, there is approximately a one in five chance that it will be missed by ACBE.
Colonoscopy
Colonoscopy may well be the most effective tool for CRC screening. colonoscopy is preferable to sigmoidoscopy, because there may be a substantial incidence of proximal colonic cancers and advanced adenomas beyond the reach of the sigmoidoscope. Advantages of colonoscopy include its usefulness in detection of >90% polyps and cancer and provides diagnosis and therapy Howevever its limitations includerisks: (Sedation/bleeding/ perforation/infections), limited availability, patient compliance and incomplete visualisation in approx 12%. A Canadian population-based study compared the risk of developing CRC after a negative colonoscopy in all Ontario residents who had a history of a complete negative colonoscopy with controls that consisted of the Ontario population without a history of colonoscopy [5]. In the negative colonoscopy cohort, the relative risk of distal CRC was significantly lower than the control group in each of the 14 years of follow-up, and the relative risk for proximal CRC was significantly lower, mainly during the last 7 years of follow-up. A second Canadian case-control study demonstrated that complete colonoscopy also was associated with fewer deaths from left-sided CRC but not from right-sided cancer [6]. Several other population-based analyses and analyses of individual screening programs in the USA, Canada, and Europe also suggest that increased use of colonoscopy is associated with mortality reduction from CRC, but that this reduction varies by site of the cancer [7-11].
Newer methods in colonoscopy: They have been suggested as a means of identifying lesions in high-risk groups or as an adjunct to colonoscopy where flat lesions (flat adenomas) are suspected. These include high-contrast endoscopy using dye or stain solutions combined with colonoscopy (chromoendoscopy), highresolution optical methods (e.g., narrow-band imaging, laser confocal endoscopy, endocystoscopy) Evidence suggests that flat or depressed neoplasms are more common than previously appreciated and that they carry a high relative risk of containing in situ or invasive carcinoma [12]. Chromoendoscopy detects more adenomas than colonoscopy using intensive inspection, but its usefulness in routine practice has not been established [13].
Virtual colonoscopy
Virtual colonoscopy uses spiral CT to generate 3D images. It requires cleaning of bowel and distension with air. It is noninvasive procedure. It has a sensitivity of 82% and specificity of around 85% [14-16]. However it is not endorsed for CRC screening. Benefits include lower risk of perforating the colon than conventional colonoscopy. Elderly patients, especially those who are frail or ill, will tolerate CT colonography better than conventional colonoscopy. For large tumours with luminal obstruction where scope cannot be negotiated beyond CT colonography will be better for visualizing proximal lesions. It is well tolerated (Sedation and pain relievers are not needed). CT colonography can detect abnormalities outside of the colon, including early-stage malignancies and potentially dangerous conditions, such as abdominal aortic aneurysms and also help in staging of malignancy, if any. However there is a small risk that inflating the colon with air could injure or perforate the bowel and bowel preparation may lead to dehydration and electrolyte imbalance. Also, there is aslight theoretical risk of developing a cancer secondary to radiation exposure. CT colonography is not recommended for pregnant women.
Several key issues need to be addressed as the use of CT colonography becomes more widespread
• Determination of the acceptable size cut-off of a lesion detected by CT colonography that will necessitate a follow-up colonoscopy.
• Need for bowel preparation,
• Ability to detect flat lesions,
• Non therapeutic
• No biopsy will be available
Carcinoembryonic antigen
CEA, may be useful in the preoperative staging and postoperative follow-up of patients with large bowel cancer, but it has a LOW PREDICTIVE VALUE for diagnosis in asymptomatic patients. The relatively low sensitivity and specificity of CEA combine to make it unsuitable for screening large asymptomatic populations.
Stool DNA: Multi-target DNA stool assay required to achieve adequate sensitivity and detect the various gene mutations stool DNA analysis has been recently cleared by FDA for colorectal screening.
Genetic testing
Genetic testing is now a reality for families with FAP. Testing for altered products of the APC gene allows early and accurate identification of family members at risk who require intensive surveillance. Proper genetic counseling, however, must be incorporated into the screening process.
FAP: It presents more difficulty than screening for FAP, because not all the genes involved have been identified, and the preferred method by which families should be screened has yet to be determined.
HNPCC: A generally accepted approach in persons with suspected HNPCC based on clinical criteria is first to perform MSI testing. Germline mutation testing for hMLH1 and hMSH2 is performed if the tumor is MSI-high, suggesting a mutation in an MMR gene. If testing for hMLH1 and hMSH2 is negative, but HNPCC still is strongly suspected, germline testing for hMSH6 can be performed.
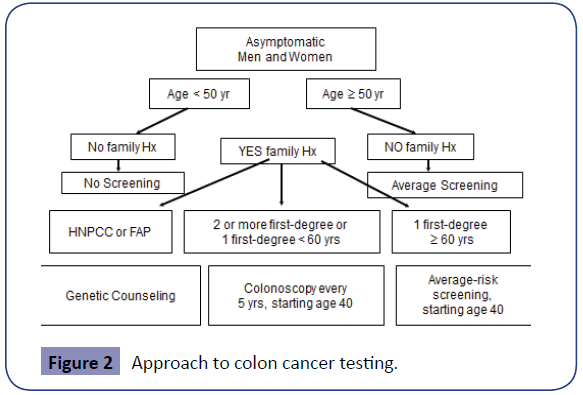
Screening Guidelines (Figure 2, Tables 2-14)
| SCREENING TOOL | U.S. PREVENTIVE SERVICES TASK FORCE * | AMERICAN CANCER SOCIETY, U.S. MULTI-SOCIETY TASK FORCE, AND AMERICAN COLLEGE OF RADIOLOGY JOINT GUIDELINES [†] |
|---|---|---|
| High sensitivity FOBT (guaiac-based or immunochemical) | Recommended annually as an option | Recommended annually as an option |
| Flexible sigmoidoscopy | Recommended every 5 yr + high-sensitivity FOBT every 3 yr as an option | Recommended every 5 yr as an option |
| Colonoscopy | Recommended every 10 yr as an option | Recommended every 10 yr as an option |
| Double-contrast barium enema | Not recommended | Recommended every 5 yr as an option |
| Computed tomographic colonography | Not recommended | Recommended every 5 yr as an option |
| Stool DNA testing | Not recommended | Recommended (interval uncertain) |
Table 2: Screening guidelines by American cancer society, U.S. Preventve services and task forces and American college of radiology [17].
| RISK CATEGORY | AGE TO BEGIN SURVEILLANCE | RECOMMENDED TESTS |
|---|---|---|
| 1 or 2 small tubular adenomas with low-grade dysplasia | 5-10 yr after initial polypectomy | Colonoscopy |
| 3 to 10 adenomas Or 1 adenoma >1 cm Or any adenoma with villous features or high-grade dysplasia |
3 yr after initial polypectomy | Colonoscopy |
| >10 adenomas on a single examination | <3 yr after initial polypectomy | Colonoscopy |
| Sessile adenomas that are removed piecemeal | 2 to 6 months to verify complete removal | Colonoscopy |
Table 3: Risk individuals.
| CRC | 3 to 6 months after cancer resection or intraoperatively | Colonoscopy | Patients with CRC should undergo high-quality perioperative clearing of the colon For nonobstructingtumors, examination can be done preoperatively; For obstructing cancer, CTC can be used to detect proximal neoplasms |
|---|---|---|---|
| Patients undergoing curative resection for CRC | 1 year after resection (or 1 year after clearing colonoscopy) | Colonoscopy | If exam at 1 year is normal, subsequent exam at 3yr. If that exam is normal, then subsequent exam at 5yr Periodic exam of the rectum (3- to 6-month intervals for the first 2-3yr) may be considered after low anterior resection of rectal cancer |
Surveillance Guidelines for People at Increased or High Risk of Colorectal Cancer (CRC)
Table 4: Screening guidelines for patients with colorectal cancer.
| Genetic diagnosis of FAP or suspected FAP without genetic testing evidence | Age 10 to 12 yr | Annual F SIG determine if the patient is expressing the genetic abnormality and counseling to consider genetic testing | If the genetic test is positive, colectomy should be considered |
FAP Screening of family members in kindreds with familial polyposis with guidelines are now available [19-22].
Table 5: Screening guidelines for FAP.
| Colorectal cancer | 100% | Colonoscopy annually, beginning age 10-12 yr |
|---|---|---|
| Duodenal or periampullary cancer | 5%-10% | Upper GI endoscopy every 1-3 yr, beginning age 20-25 yr |
| Pancreatic cancer | 2% | Possible periodic abdominal ultrasound |
| Thyroid cancer | 2% | Annual thyroid examination |
| Gastric cancer | <1% | Upper GI endoscopy as for duodenal and periampullary |
| Central nervous system cancer | <1% | Annual physical examination |
Table 6: Incidence and screening guidelines of associated malignancies in FAP.
| Genetic or clinical diagnosis of HNPCC or persons at increased risk of HNPCC | Age 20 to 25 yr or 10 yr before the youngest case in the immediate family | Colonoscopy every 1 to 2 yr and counseling to consider genetic testing | Genetic testing for HNPCC should be offered to first-degree relatives of persons with a known inherited DNA MMR gene mutation. It should also be offered when the family mutation is not known but when 1 or more of the first 3 of the modified Bethesda Criteria is present |
Table 7: Screening guidelines for HNPCC.
| Colorectal cancer | 80% | Colonoscopy, every 2 yr beginning age 20 yr, annually after age 40 yr or 10 years younger than earliest case in family |
|---|---|---|
| Endometrial cancer | 40%-60% | Pelvic exam, transvaginal ultrasound, endometrial aspirate every 1-2 yr, beginning age 25-35 yr |
| Upper urinary tract cancer | 4%-10% | Ultrasound and urinalysis every 1-2 yr; start at age 30-35 yr |
| Gallbladder and biliary cancer | 2%-18% | No recommendation |
| Central nervous system cancer | <5% | No recommendation |
| Small bowel cancer | <5% | No recommendation |
Table 8: Incidence and screening guidelines of associated malignancies in HNPCC.
| Inflammatory bowel disease (ulcerative colitis and Crohn's colitis) | Cancer risk begins to be significant 8 yr after the onset of pancolitis or 12-15 yr after the onset of left-sided colitis | Colonoscopy with biopsies for dysplasia | Every 1-2 yr. When clinically quiescent 4 quadrant biopsies every 10 cm with > 30 total samples (preferred) Additional extensive sampling of strictures and masses |
Table 9: Screening guidelines and malignancy risk for IBD.
| GI | OTHER FEATURES | MALIGNANCY RISK | SCREENING |
|---|---|---|---|
| Colorectal polyps, which may be few or resemble classic familial adenomatous polyposis | Brain tumors, including cerebellar medulloblastoma and glioblastomas | Colorectal carcinoma and brain tumors | Same as for familial adenomatous polyposis Also consider imaging of the brain |
Table 10: Screening guidelines and malignancy risk for Turcot’s Syndrome.
| GI | OTHER FEATURES | MALIGNANCY RISK | SCREENING |
|---|---|---|---|
| Polyps most commonly of colon and stomach | Mucocutaneous lesions, thyroid adenomas and goiter, fibroadenomas and fibrocystic disease of the breast, uterine leiomyomas, and macrocephaly | 10% risk for thyroid cancer and up to 50% risk for adenocarcinoma of breast in affected women | Annual physical exam with special attention to thyroid Mammography at age 30 or 5 yr before earliest breast cancer case in the family Routine colon cancer surveillance (expert opinion only) |
Table 11: Screening guidelines and malignancy risk for Cowden’s Disease.
| GI | OTHER FEATURES | MALIGNANCY RISK | SCREENING |
|---|---|---|---|
| Juvenile polyps mostly in the colon but throughout GI tract Defined by ≥ 10 juvenile polyps |
Congenital abnormalities in at least 20%, including malrotation, hydrocephalus, cardiac lesions, Meckel's diverticulum, and mesenteric lymphangioma | 9% to 25% risk for colorectal cancer; ↑ risk for gastric, duodenal, and pancreatic cancer | Screening by age 12 yr if symptoms have not yet arisen Colonoscopy with multiple random biopsies every several years (expert opinion only) |
Table 12: Screening guidelines, clinical features and malignancy risk for Familial Juvenile Polyposis.
| GI | OTHER FEATURES | MALIGNANCY RISK | SCREENING |
|---|---|---|---|
| Small number of polyps throughout GI tract but most common in small intestine | Pigmented lesions of skin; benign and malignant genital tumors | ↑ Risk for GI malignancy and pancreatic cancer and adenoma malignum of cervix; unknown risk for breast cancer | Upper GI endoscopy, small bowel radiography, and colonoscopy every 2 yr pancreatic ultrasound and hemoglobin levels annually gynecologic examination, cervical smear, and pelvic ultrasound annually clinical breast exam and mammography at age 25 yr clinical testicular exam and testicular ultrasound in males with feminizing features (expert opinion only) |
Table 13: Screening guidelines, clinical features and malignancy risk for Puetz jeghers syndrome.
| GI | OTHER FEATURES | MALIGNANCY RISK | SCREENING |
|---|---|---|---|
| Hamartomatous GI polyps, usually lipomas, hemangiomas, or lymphangiomas | Dysmorphic facial features, macrocephaly, seizures, intellectual impairment, and pigmented macules of shaft and glans of penis | Malignant GI tumors identified but lifetime risk for malignancy unknown | No known published recommendations |
Table 14: Screening guidelines, clinical features and malignancy risk for Ruvalcaba-Myhre-Smith Syndrome (Banayan-Zonana Syndrome).
Average risk individuals
No Symptoms, age< 50. no risk factors (Tables 2-14).
High risk groups (Figure 2)
Factors that put an individual at a higher risk for developing colorectal cancer include: age 60 or older, African, American or eastern European ancestry, a personal history of: colorectal cancer, cancer of the ovary, endometrium, or breast, inflammatory bowel disease (ulcerative colitis or Crohn's disease), a family history of colorectal cancer or polyps, a hereditary colorectal cancer syndrome such as familial adenomatous polyposis (FAP) or hereditary non-polyposis colon cancer (HNPCC).
Familial adenomatous polyposis and familial cancer
Screening should include genetic testing to detect abnormal APC gene products. Those who test positive should have annual or biennial flexible sigmoidoscopy, beginning at age 10 to 12 years, to assess for emergence of adenomas. If genetic testing is unavailable, annual flexible sigmoidoscopy should begin at age 10 to 12 years. Colorectal surveillance (sigmoidoscopy) should begin at age 18 to 20 years in persons with attenuated FAP (AFAP) and in persons with MUTYH mutations (in whom colonoscopy is recommended).
HNPCC
Patients with a family history of HNPCC must be examined colonoscopically, beginning at age 20 to 25 years, or at an age 10 years younger than that of the index case. A reasonable approach here is to perform colonoscopy every two years, searching for the scattered adenomas that antedate carcinomas in HNPCC; Genetic testing for HNPCC should be offered to first-degree relatives of those with a known MMR gene mutation or to those who meet the modified Bethesda criteria.
Familial CRC
The joint guidelines from the ACS recommend that ifCRC or adenomatous polyps occurred in any first-degree relative before age 60 years, in two or more first-degree relatives at any age, then colonoscopy should be performed every five years, beginning at age 40 years or beginning 10 years before the youngest case in the immediate family.
If either CRC or adenomatous polyps occurred in a first-degree relative 60 years of age or older, or if CRC occurred in two seconddegree relatives, then screening should begin at age 40 years using screening options recommended for average-risk persons. In those with more than two affected first-degree relatives, special care should be taken to exclude HNPCC, and periodic colonoscopy is advised. A recently published guidance statement from the American College of Physicians also recommends that clinicians stop screening for CRC in adults older than age 75 or in adults with a life expectancy of less than 10 years [17].
The European Guidelines for Quality Assurance in Colorectal Screening and Diagnosis is a 386-page document authored by 90 authors from 32 countries that providesan evidence-based review of existing data on CRC screening that stresses quality measures and cost-effectiveness.
HNPCC
Genetic or clinical diagnosis of HNPCC or persons at increased risk of HNPCC Age 20 to 25 yr or 10 yr before the youngest case in the immediate family Colonoscopy every 1 to 2 yr and counseling to consider genetic testing Genetic testing for HNPCC should be offered to first-degree relatives of persons with a known inherited DNA MMR gene mutation. It should also be offered when the family mutation is not known but when 1 or more of the first 3 of the modified Bethesda Criteria is present [18].
Conclusion
We should remember that time taken by a sessile polyp to get converted into pedunculated and frank adenocarcinoma is considered as golden period of time for early detection of colorectal malignancies. Even after development of malignancy in the initial stages when the disease is localized the chances of five year survival go as high as 80% and when it has distant metastasis it goes below 10% Awareness among public regarding risk factors/ symptoms using print/electronic/mass media and through national programmes, allaying discomfort or embarrassment with screening procedures, Proper infrastructure including colonoscopy labs, strategies for engaging and adequately resourcing both primary and secondary care services, proper screening and follow up of diagnosed patients including family members if indicated, are of utmost importance in colorectal screening.
References
- American Cancer Society (2011)Colorectal cancer facts & figures 2011-3. Atlanta: American Cancer Society.
- Jemal A, Bray F, Center MM (2011) Global cancer statistics.CA Cancer J Clin 61:69-90.
- Swan S, Siddiqui AA, Meyers RE (2012)International colorectal cancer screening programs: Population contact strategies, testing methods and screening rates. PractGastroenterol 20-9.
- Toma J, Paszat LF, Gunraj N, Rabeneck L(2008) Rates of new or missed colorectal cancer after barium enema and their risk factors: A population-based study. Am J Gastroenterol 103:3149-51.
- Lakoff J, Paszat LF, Saskin R, Rabeneck L (2008) Risk of developing proximal versus distal colorectal cancer after a negative colonoscopy: A population based study. ClinGastroenterolHepatol 6:1117-21.
- Baxter NN, Goldwasser MA, Paszat LF (2009) Association of colonoscopy and death from colorectal cancer. Ann Int Med 150:1-889.
- Segnan N, Patrick J, von Karsa L (2010)European guidelines for quality assurance in colorectal cancerscreening and diagnosis. Luxembourg: International Agency for Research on Cancer1-386.
- Rabeneck L,Paszat LF, Saskin R, Stukel TA(2010)Association between colonoscopy rates and colorectal cancer mortality. Am J Gastroenterol105:1627-32.
- Brenner H, Hoffmeister M, Volker A (2010) Protection from right-and left-sided colorectal neoplasms aftercolonoscopy: Population-based study. J Natl Cancer Inst 102:89-95.
- Stock C, Knudsen AB, Lansdorp-Vogelaar L (2011)Colorectal cancer mortality prevented by use and attributable to colonoscopy. GastrointestEndosc 73:435-43.
- Singh H, Nugent Z, Demers AA (2010)The reduction in colorectal cancer mortality after colonoscopy varies by siteof cancer. Gastroenterology 139:1128-37.
- Kahi CJ, Imperiale TF, Juliar BE, Rex DK (2009) Effect of screening colonoscopy on colorectal cancer incidence andmortality. ClinGastroenterol7:770-5
- Soetikno RM, Kaltenbach T, Rouse RV (2008) Prevalence of nonpolypoid (flat and depressed) colorectal neoplasms inasymptomatic and symptomatic adults. JAMA 299: 1027-1035.
- Stoffel EM, Turgeon DK, Stockwell DH (2008)Chromoendoscopy detects more adenomas than colonoscopy using intensive inspection without dye spraying. Cancer Prev Res 1:507-13.
- Akerkar GA, Yee J, Hung R, McQuaid K (2001) Patient experience and preferences toward colon cancer screening: a comparison of virtualcolonoscopy and conventional colonoscopy. GastrointestEndosc 54:310–315.
- Fenlon HM, Nunes DP, Schroy PC, Barish MA, Clarke PD, et al. (1999) A comparison of virtual and conventional colonoscopy for the detection of colorectal polyps. N Engl J Med 341:1496–1503.
- Pickhardt PJ, Choi JR, Hwang I (2003) Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med 349:2191–2200.
- Zauber AG, Landsdorp-Vogelaar I, Knudsen AB (2008) Evaluating test strategies for colorectal cancer screening: A decision analysis for the U.S. Preventive Services Task Force. Ann Intern Med149:659-69.
- Quaseem A, Denberg TD, Hopkins RH Jr (2012) Screening for colorectal cancer: A guidance statement from the American College of Physicians. Ann Intern Med 156:378-86.
- Vasen HF, Moslein G, Alonso A (2008) Guidelines for clinical management of familial adenomatous polyposis (FAP). Gut 57:704-13.
- Burt RW, Barthel JS, Dunn KB (2010) NCCN clinical practice guidelines in oncology.Colorectal cancer screening. J NatlComprCancNetw 8:8-61.
- Bresalier R (2010) Management of high-risk colonoscopy patients. GastrointestClin North Am 20:629-40.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences