Small Cell Carcinoma of the Rectum, A Systematic Literature Review and Case Series
Qasem E, Nangalia M, Jones H, Griffiths P, Sekaran C, Evans MD, Davies M, Khot U, Beynon J, Williams N and Harris DA
Qasem E1, Nangalia M1, Jones H1, Griffiths P2, Sekaran C1, Evans MD1, Davies M1, Khot U1, Beynon J1, Williams N2 and Harris DA1
1Department of Surgery, Morriston Hospital, Swansea, UK
2Department of Pathology, Singleton Hospital, Swansea, UK
- *Corresponding Author:
- Qasem E
Department of Colorectal Surgery, Abertawe Bro Morgannwg University Local Health Board, Morriston Hospital, Swansea SA6 6NL, UK
Tel: 01792-205110
E-mail: eyasqasem@doctors.org.uk
Received date: November 24, 2015; Accepted date: January 02, 2016; Published date: January 08, 2016
Citation: Qasem E, Nangalia M, Jones H, et al. Small Cell Carcinoma of the Rectum, a Systematic Literature Review and Case Series. Colorec Cancer. 2016, 2:1. doi: 10.21767/2471-9943.100010
Abstract
Small cell carcinoma (SCC) is a rare malignancy that can affect the gastrointestinal tract. Current knowledge is limited to individual case series. Here we present three cases from our own institution and report the results of a systematic review of the literature.
Methods and findings: A ten-year review of our institutions pathology database for rectal SCC was performed. A systematic search of Embase, PubMed and Google Scholar with cross-referencing was performed applying suitable exclusion criteria. Three cases were identified in our institution from 2004-2014. All were positive for synaptophysin, TTF-1, CD56 and CK positive. All had metastatic disease at presentation and had chemoradiotherapy. Median survival was 13 months. A systematic search found 112 articles of which 41 were included (121 patients). Optimal survival (37 months) was observed with a combination of surgery, chemotherapy and radiotherapy.
Conclusion: SCC rectum is a rare entity that is usually metastatic at presentation and has poor long-term survival despite aggressive treatment schedules. Prospective registries would optimize management of this unusual neuroendocrine tumour.
Keywords
Small cell carcinoma; Rectal cancer
Introduction
Small cell carcinomas (SmCC) are malignancies that derive from neuroendocrine cells. The World Health Organisation (WHO) in 2010 classified SmCC as a subgroup of neuroendocrine carcinoma (NEC) which accounts for approximately 0.6 percent of all colorectal cancers [1]. NEC has two histologic subgroups, namely large cell carcinoma (LCC) and small cell carcinoma (SmCC). LCC can be difficult to distinguish from poorly differentiated adenocarcinoma [2].
Despite the fact that the gastrointestinal tract (GI) has the largest number of neuroendocrine cells in the body it is unusual for such malignancies to occur here [3]. First described in 1952, nearly 650 cases of gastrointestinal SmCC have been reported in the literature until 2007 [4]. The oesophagus is the commonest site of incidence of GI SmCC (53%) followed by the colon (13%), stomach (11%), gallbladder (8.4%) and rectum (7.3%). The aggressive neoplastic nature of SmCC is characterised by rapid growth, high mitotic proliferation rate (>20 mitotic figures per 10 high-power fields [HPF] or a Ki-67 index >20%), early dissemination and poor prognosis [1-5].
SmCC of the rectum is an extrapulmonary small cell carcinoma (EPSmCC) entity, which morphologically resembles pulmonary small cell carcinoma (PSmCC). SmCC was originally thought to derive from amine-precursor uptake and decarboxylase cells (APUD). However, the pluripotent stem cell, with its potential for variable differentiation, is now the most widely accepted theory [6-9]. Although it is important to rule out a PSmCC as the primary tumour the treatment paradigm for EPSmCC is extrapolated form the platinum based therapy used to treat PSmCC [10-12].
The majority of the available literature for rectal or even GI SmCC is constituted of case reports and case series. Therefore, the objectives of the study are to describe the presentation, investigations and management of 3 patients presenting with rectal SmCC to our institution, and to systematically review this rare entity in reported literature looking into survival as a primary outcome and treatment strategies.
Method
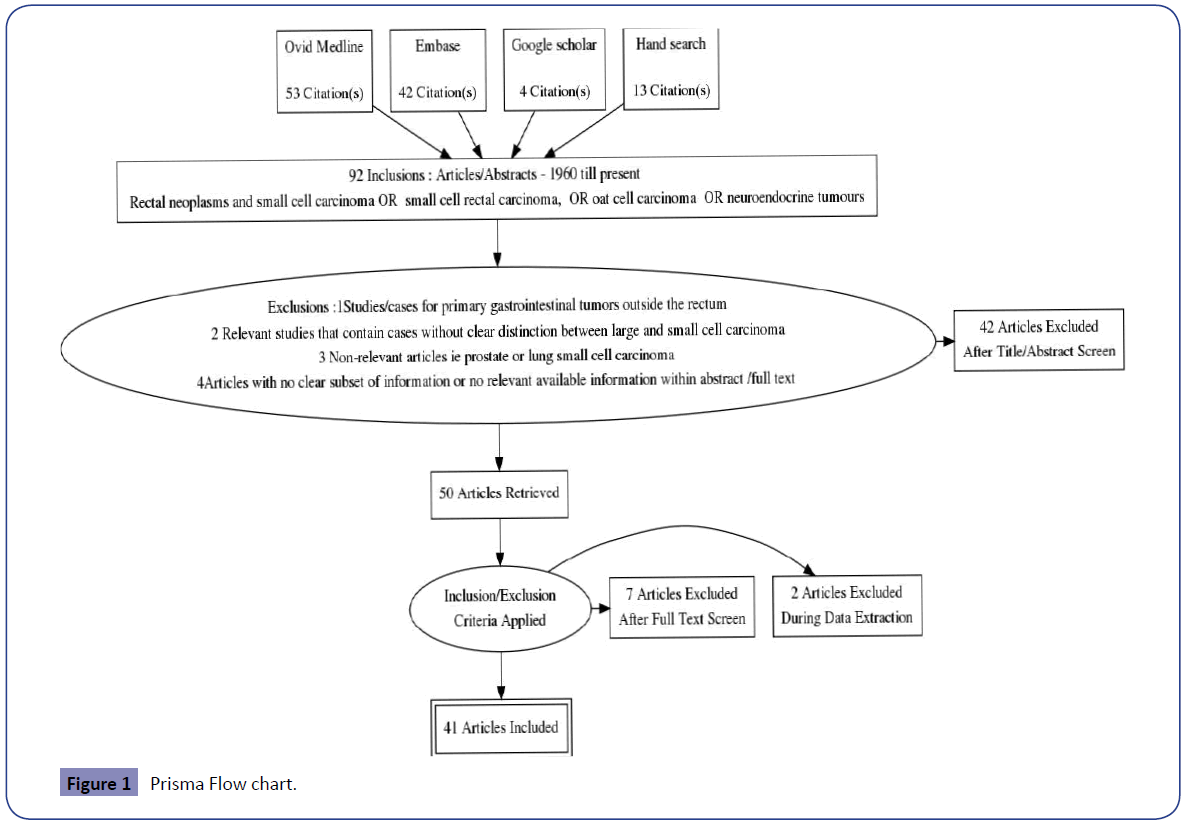
A systematic review was conducted to identify studies of all patients diagnosed with SmCC of the rectum in line with PRISMA methodology. The main outcome was to evaluate the overall survival for the reported treatment modalities. This was performed by electronic database interrogation by two experienced reviewers using a formal assessment of methodological quality; including manual searches of reference lists of relevant articles and consultation with experts in the field. No limits were applied except for English Language.
We searched the Embase, Ovid MEDLINE, Cochrane and Google Scholar databases for publications with the final search being conducted on November 13th 2015. We searched for the following terms: Rectal neoplasms AND small cell carcinoma OR “ small cell rectal carcinoma”, OR “oat cell carcinoma” OR “neuroendocrine tumours”.
Exclusions
1. Studies/cases for primary gastrointestinal tumours outside the rectum.
2. Relevant studies that contain cases without clear distinction between large and small cell carcinoma.
3. Non-relevant articles i.e., prostate or lung small cell carcinoma.
4. Articles with no clear subset of information or no relevant available information within abstract /full text.
A critical appraisal of included case series using the National Institute for Health and Care Excellence Quality Assessment for Case Series Form (Table 1) was included which scores study quality out of a maximum possible score of 8.
| Author | Qualitative score 71 (Median 3, range 1-7) |
|---|---|
| Modrek | 7 |
| Demellawy | 5 |
| Gaffey | 4 |
| Staren | 2 |
| Cebrian | 1 |
| Shirouzu | 1 |
Table 1: Quality Assessment for Case Series.
Data extraction was done for each article, which divided cases into treatment modalities (Table 2). Surgical treatment was defined as “Extended surgery”, including codes for rectal amputation, abdominoperineal resection (APR), complete proctectomy with/without lymphadenectomy or lymph node dissection, and surgeries with partial or total removal of other adjacent organs. “Local surgery” includes codes for: local tumour excision (such as transanal excisions), anterior resection, segmental resection, or surgery not otherwise stated (NOS) [13].
| Treatment Modality | Subgroup | Number of patients | Stage IV * | Median survival (range) |
|---|---|---|---|---|
| Surgery | Extended | 32 | 40.6 % | 9 mo (2-84) |
| Surgery | 15 | 40% | 7 mo (2-84) | |
| +CTX | 6 | 83% | 16 mo (4-48) | |
| + RTX | 7 | NA | NA | |
| +CTX+RTX | 4 | 25% | 10(4-16) | |
| Local | 25 | 27% | 6 (3-18) | |
| Surgery | 11 | 36% | 6(2-10) | |
| +CTX | 4 | 27% | 7.5(3-9) | |
| +RTX | 7 | 0 % | NA | |
| +CTX+RTX | 3 | 50% | 30 (18-42) | |
| Chemotherapy (CTX)** | +Surgery | 10 | 80% | 9(3-48) |
| Chemotherapy Regimen | ETP+CIS | 13 | 61.5% | 6.5 (2-48) |
| Other ** | 15 | 73 % | 9 (2-36) | |
| Radiotherapy (RTX) | +Surgery | 16 | 12.5% | NA |
| +Chemotherapy | 6 | 66% | 12(3-72 | |
| +Surgery + Chemotherapy | 5 | 40% | 17 (4-42) |
*At presentation
**Cisplatin/Etoposide/Folfox/Vincristine/Cyclophosphamide/Carboplatin/Irenotecan/Methotrexate/Bevacizumab/Doxorubicin (different combinations) [9,13,18,22-29,32,33,41-71].
Table 2: Systematic Review Results.
Patients were then classified into outcomes for chemotherapy in addition to surgery. The Etoposide (ETP) and Cisplatin (CIS) regimen was most frequently reported, followed by various combinations of chemotherapeutic agents, some of which included CIS and ETP. Lastly we included patients having radiotherapy in combination with surgery and/or chemotherapy.
In addition to the systematic literature review we evaluated the experience of our University hospital in the management of rectal SmCC. A consultant pathologist conducted a retrospective review of the hospital pathology database. This cross-referenced rectal cancer cases and SmCC over a ten-year study period (2004-2014). Once pathologically verified the paraffin embedded slides were dewaxed and stained with Synaptophysin, TTF1, Chromogranin, CD56, CD20, CD7 and CK antibodies (Ventana, Inc) for immunohistochemistry.
Using the Patient Information Management System (PIMS), the corresponding investigations, management and survival data were retrieved.
Results
112 articles were identified (52 Ovid MEDLINE, 43 Embase, 4 Google Scholar and 13 Hand searches). 22 were duplicates and 50 studies were excluded as shown in Figure 1.
Two authors (EQ, MN) reviewed abstracts and then full texts of all included 41 studies and crosschecked suitability for inclusion – none were randomised clinical trials.
190 patients (cases) of rectal small cell rectal cancer were identified; only 121 patients had sufficient data to be included in the analysis. The age range was (24-78 years). Forty two percent of patients were female. The studies showed considerable variation in the amount of reported data. The main fields of interest were gender, age at diagnosis, treatment modality, presence of metastases at presentation and survival. Missing data was recorded.
Neither detailed TNM staging nor histological grading was available in the majority of studies. 6 case series were assessed against the NICE guidelines qualitative questionnaire.
The patients who had radiotherapy, chemotherapy and surgery had the best survival (37 months). The individuals that underwent “Extended surgery” had relatively better survival than those who had “Local surgery” but they were more likely to have metastasis at presentation (40.6% vs. 27%). Patients who were treated with triple (Surgery +CTX +RTX) therapy were more likely to survive compared to patients that received single or dual modalities.
Despite the fact that the median survival for the chemotherapy plus surgery group was just 9 months and more likely to have distant disease at presentation (80%), some studies reported individual patients having survival figures in excess of 70 months (data not shown).
Although the number of included patients was sufficient the small sample size for different treatment groups as well the inconsistent recording of data limited the ability for statistical power. Hence, a meta-analysis was not deemed appropriate.
Retrospective review of our institutions pathology database over the last 10 years identified 3 patients (2 females, 1 male). All patients were treated with chemotherapy (Cisplatin and Etoposide) followed by consolidation radiotherapy. None had any surgical intervention. The median survival was 13 months (Table 3). Immunostaining results with Synaptophysin, TTF1, Chromogranin CK7, CK20, CD56 is shown in Table 4. All were TTF1 and CK positive.
| Patient | Age | G | Surgery | Chemoradio therapy (Cisplatin+Etoposide) | Radiotherapy | Survival(Months) | Metastasis | Cause of death |
|---|---|---|---|---|---|---|---|---|
| A | 62 | F | N | Y | Y | 13 | Y | DOD |
| B | 69 | F | N | Y | Y | 28 | Y | DOD |
| C | 69 | M | N | Y | Y | 6 | Y | DOD |
Table 3: Demographics, treatment modality and survival for our patient series.
| Synaptophysin | TTF1 | Chromogranin | CD56 | CD20 | CD 7 | CK | |
|---|---|---|---|---|---|---|---|
| A | +++ | +++ | - | +++ | + (Very focal) | +++ | +++ |
| B | +++ | +++ | +(Weak,focal) | +++ | - | +(Very focal) | +++ |
| C | ++ | ++(focal) | - | +++ | - | - | +++ |
Table 4: Immunohistostaining for our patient series.
Discussion
Small cell carcinoma (SmCC) falls under the umbrella of extrapulmonary small cell carcinoma (EPSmCC), which is a group of uncommon endocrine neoplasms that has been reported to involve any organ. SmCC resembles pulmonary small cell carcinoma (PSmCC), a common primary lung cancer, which contributes to nearly a quarter of lung cancer [13,14]. SmCC was thought to derive from neuroendocrine cells amine-precursor uptake and decarboxylase (APUD) [15]. However, Pluripotent stem cells with the potential for variable differentiation to be the precursor is the most widely accepted theory [8,16].
Duguid reported the first EPSmCC in 1930. Twenty-two years later Mckeown reported the first gastrointestinal case. Since then more than 500 cases had been reported. Approximately 200 cases of rectal small cell carcinoma were reported until present. The rectum if the most common location of small cell carcinoma in the large bowel followed by the caecum and sigmoid colon [17].
The available literature consists mainly of series of case reports or small case series [18]. Prognosis is very poor for these patients that can be secondary that the tumor’s biology is aggressive, metastatic disease at the time of diagnosis, with poor response to chemo/radiotherapy.
Seventy percent of patients present with metastases at the time of diagnosis [19,20] The reported 6 months, 3 year and 5 year survival rates of 58%, 13% and 6% respectively, with a median survival of 10.4 months [21]. The systematic review conducted here found a median survival of 8.5 months for all included studies.
SmCC is a distinct clinicopathological entity. The fact that SmCC resembles the PSmCC undermines the importance of excluding a primary lung tumour with secondary metastasis to the GI tract. Other differential diagnoses include cloacogenic carcinoma, lymphoma, embryonic rhabdomyosarcoma, amelanotic melanoma and carcinoid.
Peak incidence of these tumours is in the sixth and seventh decades of life. The limited information on colorectal small cell carcinoma in the English literature shows that these tumours have similar demographic features to colorectal adenocarcinomas apart from this being more common in females that contradict our findings of an increased male predominance.
The data extrapolated from Survey of Epidemiology and End Results (SEER) reported several patient characteristics associated with colorectal NEC (1367 patients) as compared to colorectal adenocarcinoma. Neither smoking nor poverty were associated with colorectal NEC. The incidence was significantly different amongst different races/ethnicities, with the lowest incidence in white Hispanic and Asian populations [22].
The clinical presentation of this unusual tumour mimics the usual presentation of other colorectal malignancies without any distinctive features. Occasional correlations and rare presentations have been reported. Small cell carcinoma rarely presented as paraneoplastic syndrome [23] but a number of rectal SmCC case reports demonstrated an association with chronic ulcerative colitis, although adenocarcinoma is the malignancy most commonly associated with a background of ulcerative colitis [24–27]. HIV has also been correlated as well as HPV with rectal SmCC [28–30].
Classically the patients will have similar workup (i.e., Pelvic MRI, CT scan of the chest, abdomen and pelvis) to other rectal cancers until the histology is confirmed.
Some authors suggest routine bronchoscopy to rule out a primary SmCC. Investigators also advise to perform a bone marrow biopsy if there are features of bone marrow involvement such as thrombocytopenia, or peripheral blood leukoerythroblastosis levels [3].
Positron emission tomography is a suitable staging adjunct, taking into consideration the high possibility of a metastatic disease. The use of radiolabelled somatostatin analog scans in GI SmCC is restricted to a small case series and as such is not currently recommended as standard of care. However, exploring its potential seems appropriate as this scan is frequently used in other gastrointestinal low grade neuroendocrine tumours [3,4].
Rectal SmCC appears similar to the equivalent pulmonary malignancy, having scanty cytoplasm, fusiform cell shape, fine granular chromatin, absent or small nucleoli, and nuclear molding [3,31,32].
Immunohistochemistry truly differentiates small cell carcinoma as a distinctive entity. Tumour cells tend to be positive for CD56 (neural cell adhesion molecule), which was confirmed in our case series, presented here. CDX2, mCEA, synaptophysin, neuronespecific enolase, keratin, and chromogranin immunohistochemistry can help differentiate it from non-endocrine poorly differentiated adenocarcinoma [17,32-34].
SmCC samples stained positive for CD56 significantly more than those of SYN, CgA, EMA, CK, and NSE (P<0.001) [34]. The expression of EGFR was positive in the majority of colorectal SmCC patients [32]. Staining for thyroid transcription factor 1 (TTF-1) is reported to be uniformly negative in rectal SmCC [32,35], in contrast to pulmonary and other extrapulmonary SmCC [36]. This was contradicted in our small series, as all of the rectal SmCC were TTF-1 positive.
PSMCC and GI SmCC are similar at a cellular level with shared molecular characteristics, which include high proliferative activity, prevalence of p53 overexpression, Rb loss and telomerase activation, and rarity of p16 loss and k-r‘ m s mutations. Brenner et al. [18] suggested that there are some disparities of importance, one of which are the efficacy of surgery for example in SMCC compared to PSMCC. Although Brenner identified “pure” small cell carcinoma as an entity, response to treatment was not differentiated from other groups [18].
Treatment guidelines for GI SmCC is decided by fundamentally extrapolating from the much more recognised entity of primary PSmCC using platinum based chemotherapy and the use of surgical resection and adjuvant chemotherapy (colorectal adenocarcinoma treatment) for specifically colorectal SmCC [2,3,11]. Therefore the treatment algorithm for PSmCC suggests that treatment of choice should be multi-modality for limited disease and systemic chemotherapy for extensive disease [37].
This concords earlier reports which have indicated that radical surgery in isolation is inadequate to control local extrapulmonary small cell carcinoma due to the high relapse rates and short disease-free survival time [38]. The combination of adjuvant chemo-radiotherapy after definitive surgery for limited disease extrapulmonary small cell carcinoma has been associated with superior outcomes [39].
In a relatively large sample study including 126 high-grade neuroendocrine colorectal carcinomas (large and small cell), the surgical approach used to resect the primary tumour was not associated with better outcomes in patients presenting with either localised or metastatic disease [40]. This was also supported by a study looking into NEC within the SEER registry (populationbased Survey of Epidemiology and End Results registry) data, which demonstrated in minimal difference in relative survival for patients with non-metastatic compared with metastatic colorectal small cell carcinoma [22].
An article reporting the largest series of small cell carcinoma of the rectum (73 patients) found that there was a significant survival advantage for localised small cell rectal cancer treated with radiotherapy compared to those who did not receive radiotherapy [13]. Whether the patients had chemotherapy or not was not described. Although this study contributed a large number of patients to our study, no overall survival data were presented for different subsets so we could not report this in our results.
Although the authors in collaboration with experienced academic librarians used the most commonly utilised databases for the literature search, we acknowledge that source selection bias, publication bias as well as language bias can affect our results.
Prospective clinical trials for efficacy of treatment modalities are not possible for rare malignancies such as rectal SmCC. Therefore, accurate histological diagnosis and “tailored” treatment options should be determined in a multidisciplinary manner in order to achieve optimal outcomes. A prospectively maintained international registry for this relatively rare malignancy would help determine optimal management strategy.
Conclusion
This is the first systematic literature review of SmCC of the rectum. There is no agreed standard treatment approach for this aggressive colorectal SmCC at the present time. An optimal survival result from combined surgery, chemotherapy and radiotherapy but overall prognosis is poor.
References
- Bosman F, Carneiro F, Hruban R, Theise N(2010) WHO classification of tumours of the digestive system.
- Sorbye H, Strosberg J, Baudin E, Klimstra DS, Yao JC (2014)Gastroenteropancreatic high-grade neuroendocrine carcinoma. Cancer120:2814–2823.
- Brenner B, Tang LH, Klimstra DS, Kelsen DP (2004) Small-cell carcinomas of the gastrointestinal tract: a review J Clin Oncol22:2730–2739.
- Brenner B, Tang LH, Shia J, Klimstra DS, Kelsen DP(2007) Small cell carcinomas of the gastrointestinal tract: clinicopathological features and treatment approach. Semin Oncol 34:43–50.
- Clery AP, Dockerty MB, Waugh JM (1961) Small-cell carcinoma of the colon and rectum. A clinicopathologic study. Arch Surg2:164–172.
- Remick S, Ruckdeschel J (1992) Extrapulmonary and pulmonary small-cell carcinoma: Tumor biology, therapy, and outcome. Medical and Pediatric Oncology. Wiley20.
- Hoang, Maitra, Gazdar, Albores-Saavedra (2001) Primary mammary small-cell carcinoma: a molecular analysis of 2 cases. Human Pathology32: 753-757.
- Ichimura H, Sakashita N, Iida T, Chisaka T, Yasuda H, et al. (1999) Differentiation of a cell line of human cervical argyrophil small cell carcinoma to macrophage lineage cells. Jpn J Cancer Res 90:523–9.
- Richardson RL, Weiland LH (1982) Undifferentiated small cell carcinomas in extrapulmonary sites. Semin Oncol9:484–496.
- Moertel CG, Kvols LK, O’Connell MJ, Rubin J (1991) Treatment of neuroendocrine carcinomas with combined etoposide and cisplatin. Evidence of major therapeutic activity in the anaplastic variants of these neoplasms. Cancer 68:227–232.
- Strosberg J, Coppola D, Klimstra D, Phan A, Kulke M, et al (2010) The NANETS Consensus Guidelines for the Diagnosis and Management of Poorly Differentiated (High-Grade) Extrapulmonary Neuroendocrine Carcinomas. Pancreas39.
- Rindi G, Inzani F, Solcia E (2010) Pathology of gastrointestinal disorders. Endocrinol Metab Clin North Am. 39:713–727.
- Modrek AS, Hsu HC, Leichman CG, Du KL (2015) Radiation therapy improves survival in rectal small cell cancer - Analysis of Surveillance Epidemiology and End Results (SEER) data. Radiation oncology (London, England) 10:101.
- BarnardWG (1926) The nature of the “oat-celled sarcoma” of the mediastinum. The Journal of Pathology and Bacteriology 29: 241–244.
- MorganLC, GraysonD, PetersHE (2000) Lung cancer in New South Wales: current trends and the influence of age and sex. The Med J Aust172:578-582.
- Pearse AG (1969) The cytochemistry and ultrastructure of polypeptide hormone-producing cells of the APUD series and the embryologic, physiologic and pathologic implications of the concept. J Histochem Cytochem 17:303–313.
- Hoang MP, Maitra A, Gazdar AF, Albores-Saavedra J (2001) Primary mammary small-cell carcinoma: a molecular analysis of 2 cases. Hum Pathol 32:753–757.
- Cebrian J, Larach SW, Ferrara A, Williamson PR, Trevisani MF, et al. (1999) Small-cell carcinoma of the rectum: report of two cases. Dis Colon Rectum 42:274–277.
- Brenner B, Shah MA, Gonen M, Klimstra DS, Shia J, et al. (2004) Small-cell carcinoma of the gastrointestinal tract: a retrospective study of 64 cases. Br J Cancer90:1720–1726.
- Bernick PE, Klimstra DS, Shia J, Minsky B, Saltz L, et al. (2004) Neuroendocrine carcinomas of the colon and rectum. Dis Colon Rectum 47:163–169.
- Burke AB, Shekitka KM, Sobin LH (1991) Small cell carcinomas of the large intestine. Am J Clin Pathol. 95:315–21.
- Ihtiyar E, Algin C, Isiksoy S, Ates E (2005) Small cell carcinoma of rectum: a case report. World J Gastroenterol.20:3156–3158.
- Vergelí-Rojas JA, Santiago-Caraballo DL, Cáceres-Perkins W, Magno-Pagatzartundua P, Toro DH (2013) Small cell neuroendocrine carcinoma of rectum with associated paraneoplastic syndrome: a case report. P R Health Sci J32:51–53.
- Yaziji H, Broghamer WL (1996) Primary small cell undifferentiated carcinoma of the rectum associated with ulcerative colitis. South Med J 89:921–4.
- Kosmidis C, Efthimiadis C, Anthimidis G, Vasiliadou K, Tzeveleki I, et al. (2010) Small cell carcinoma in ulcerative colitis - new treatment option: a case report. World Journal of Surgical Oncology 8:100.
- Hayashi H, Miyagi Y, Sekiyama A, Yoshida S, Nakao S, et al. (2012) Colorectal small cell carcinoma in ulcerative colitis with identical rare p53 gene mutation to associated adenocarcinoma and dysplasia. J Crohns Colitis. 6:112–115.
- Rubin A, Pandya PP (1990) Small cell neuroendocrine carcinoma of the rectum associated with chronic ulcerative colitis. Histopathology 16:95–97.
- Smitherman MH, Morris LE, Chang BK, Khankhanian NK, Dunlap DB (1990) Rectal small cell cancer in an HIV-positive man. Am J Med 89:239–240.
- Read EJ, Orenstein JM, Chorba TL, Schwartz AM, Simon GL, et al. (1985) Listeria monocytogenes sepsis and small cell carcinoma of the rectum: an unusual presentation of the acquired immunodeficiency syndrome. Am J Clin Pathol 83:385–389.
- Cimino-Mathews A, Sharma R, Illei PB (2012) Detection of human papillomavirus in small cell carcinomas of the anus and rectum. Am J Surg Pathol 36:1087–1092.
- Joshua AM, Adams D, McKenzie P, Solomon M, Clarke SJ (2005) Small blue cell tumors of the rectum. Case 2. Small-cell carcinoma of the rectum. J Clin Oncol23: 912–913.
- Demellawy D, Khalifa M, Ismiil N, Wong S, Ghorab Z (2007)Primary colorectal small cell carcinoma: A clinicopathological and immunohistochemical study of 10 cases. Diagnostic Pathology 2:35.
- Kanat O, Qzet A, Ataergin S, Komurcu S, Onguru O (2006) Small cell carcinoma of the large bowel: a rare, but very aggressive malignancy. Am J Gastroenterol 101:2440–2441.
- YunJP, XiangJ, HouJH, TianQH (2005) Expression of CD56, as a potential diagnostic marker, in small cell carcinoma. Ai Zheng24:1140-1143.
- OrdóñezNG (2000) Value of thyroid transcription factor-1 immunostaining in distinguishing small cell lung carcinomas from other small cell carcinomas. Am J Surg Pathol24:1217-1223.
- KaufmannO, DietelM (2000) Expression of thyroid transcription factor-1 in pulmonary and extrapulmonary small cell carcinomas and other neuroendocrine carcinomas of various primary sites. Histopathology36: 415-420.
- Chalermchai T, Suwanrusme H, Chantranuwat P, Voravud N, Sriuranpong V (2010) Retrospective review of extra-pulmonary small cell carcinoma at King Chulalongkorn memorial hospital cases during 1998-2005. Asia Pac J Clin Oncol 6:111–115.
- Kim, Lee, Chun, Shin (2006) Clinical overview of extrapulmonary small cell carcinoma. J Korean Med Sci21: 833-7.
- Kim K-OO, Lee H-YY, Chun S-HH, Shin S-JJ, Kim M-KK, et al. (2006) Clinical overview of extrapulmonary small cell carcinoma. J Korean Med Sci 21:833–837.
- Smith J, Reidy D, Goodman K, Shia J, Nash G (2014) A Retrospective Review of 126 High-Grade Neuroendocrine Carcinomas of the Colon and Rectum. Annals of Surgical Oncology 21:2956–2962.
- Shafqat H, Ali S, Salhab M, Olszewski AJ (2015) Survival of patients with neuroendocrine carcinoma of the colon and rectum: a population-based analysis. Dis Colon Rectum 58:294–303.
- Al-Jiffry BO, Al-Malki O (2013)Neuroendocrine small cell rectal cancer metastasizing to the liver: a unique treatment strategy, case report, and review of the literature. World J Surg Oncol11:153.
- Vilor M, Tsutsumi Y, Osamura RY, Tokunaga N, Soeda J, et al. (1995) Small cell neuroendocrine carcinoma of the rectum. Pathol Int 45:605–609.
- Okuyama T, Korenaga D, Tamura S, Yao T, Maekawa S, et al. (1999)The effectiveness of chemotherapy with cisplatin and 5-fluorouracil for recurrent small cell neuroendocrine carcinoma of the rectum: report of a case. Surg Today 29:165–169.
- Izuishi K, Arai T, Ochiai A, Ono M, Sugito M et al. (2002) Long-term survival in advanced small cell carcinoma of the colorectum: report of a case. Surg Today 32:72–74.
- Shirouzu K, Morodomi T, Isomoto H, Yamauchi Y, Kakegawa T et al. (1985) Small-cell carcinoma of the rectum. Clinicopathologic study. Dis Colon Rectum 28:434–439.
- Sakamoto Y, Kitajima Y, Ogawa A, Hidaka K, Miyazaki K (1999) Successful combination chemotherapy for a case of small cell carcinoma of the rectum with multiple liver metastasis. Gan To Kagaku Ryoho 26:543–547.
- Tuncbilek, Karakas, Okten (2006) Use of dynamic contrast-enhanced magnetic resonance imaging for differentiating between aggressive rectal tumours: two cases with small cell carcinoma and malignant melanoma. Hong Kong Med J12:480-482.
- Tokatli, Koçak, Ozyilmaz, Uygun (2002) Small cell carcinoma of the rectum; report of a case.J B U7: 75-77
- Palvio DH, Sørensen FB, Kløve-Mogensen M (1985) Stem cell carcinoma of the colon and rectum. Report of two cases and review of the literature. Dis Colon Rectum 28:440–445.
- Shirouzu K, Morodomi T, Isomoto H, Ono S, Kakegawa Tet al.(1987) Long term survival case of small (oat) cell carcinoma of the rectum. Acta Pathol Jpn 37:111–116.
- Schwartz AM, Orenstein JM (1985) Small-cell undifferentiated carcinoma of the rectosigmoid colon. Arch Pathol Lab Med 109:629–632.
- Wu Y, Wang Q, Wang L, He X (2012) Extrapulmonary Small Cell Carcinoma of the Rectum. J Gastrointest Cancer 1:258–261.
- Izik O (2010) Small cell carcinoma of the rectum metastasizing to axillary lymph nodes: A case report. Turkish Jn Gastro21: 476-477.
- Kuratate S, Inoue S, Chikakiyo M, Kaneda Y, Harino Yet al.(2010) Coexistent poorly-differentiated neuroendocrine cell carcinoma and non-invasive well-differentiated adenocarcinoma in tubulovillous adenoma of the rectum: report of a case. J Med Invest57: 338–344.
- Sato K, Yokouchi Y, Saida Y, Ito S, Kitagawa T et al.(2012) A small cell neuroendocrine carcinoma of the rectum diagnosed by colorectal endoscopic submucosal dissection. Journal of gastrointestinal and liver diseases: JGLD.21:128.
- SatoY, FujisawaJ, SajiY, MisawaK (1992) A case of small cell undifferentiated carcinoma (SCUC) of the rectum treated with etoposide, cis- platinum and radiotherapy]. Gan to Kagaku Ryoho19: 2245-2249.
- Kuratate S, Inoue S, Chikakiyo M, Kaneda Y, Harino Y et al. (2010) Coexistent poorly-differentiated neuroendocrine cell carcinoma and non-invasive well-differentiated adenocarcinoma in tubulovillous adenoma of the rectum: report of a case. J Med Invest57: 338–344.
- Mohammad A, Varghese T, Shanbag V, Soma S. Small Cell Carcinoma Rectum With Liver Metastases: A Case Report.JDMS13: 101-102
- Murphy JM, Daly P, Wilson GF(1999) Endorectal ultrasound in a small cell carcinoma of the rectum; staging and assessment of response to chemotherapy. Clin Radiol 54:696–698.
- Ree AH (2006) A complex case of rectal neuroendocrine carcinoma with terminal delirium. Nat Clin Pract Gastroenterol Hepatol 3:408–413.
- Hussein AM, Feun LG, Sridhar KS, Otrakji CL, Garcia-Moore M, et al. (1990) Small cell carcinoma of the large intestine presenting as central nervous systems signs and symptoms. Two case reports with literature review. J Neurooncol 8:269–274.
- Khansur TK, Routh A, Mihas TA, Underwood JA, Smith GF et al. (1995) Syndrome of inappropriate ADH secretion and diplopia: oat cell (small cell) rectal carcinoma metastatic to the central nervous system. Am J Gastroenterol 90:1173–1174.
- D’souza A, Dea S, Karunasiri D (2014) A curious case of small cell carcinoma . J gen intern medConference 37th Annual Meeting of SGIM ,San Diego , CA United states 29: S273-S274 .
- Spiliopoulou P, Panwar U, Davidson N (2011) Rectal small cell carcinoma: a case report and review of the literature. Case Rep Oncol 4:475–480.
- Gaffey MJ, Mills SE, Lack EE (1990) Neuroendocrine carcinoma of the colon and rectum. A clinicopathologic, ultrastructural, and immunohistochemical study of 24 cases. Am J Surg Pathol14:1010–1023.
- Yalcin S, Ozsik Y, Tekuzman G (1998) Metastatic small-cell carcinoma of rectum responding dramatically to combination chemotherapy. Am j clin oncol 21:75–76.
- Robidoux A, Monte M, Heppell J, Schurch W (1985) Small-cell carcinoma of the rectum. Dis Colon Rectum28:594–596.
- SakamotoY, KitajimaY, OgawaA(1999)Successful combination chemotherapy for a case of small cell carcinoma of the rectum with multiple liver metastasis. Gan To Kagaku Ryoho26: 543-547
- Staren ED, Gould VE, Warren WH, Wool NL, Bines Set al.(1998) Neuroendocrine carcinomas of the colon and rectum: a clinicopathologic evaluation. Surgery 104:1080–1089.
- https://www.nice.org.uk/guidance/cg3/resources/appendix-4-quality-of-case-series-form2.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences